Poly (lactic-co-glycolic acid) (PLGA) has become a front-runner in the development of modern drug delivery systems (DDSs) due to its remarkable biocompatibility, biodegradability, and FDA approval for human use. Despite its popularity, conventional bulk synthesis techniques often suffer from limitations such as low drug loading efficiency, broad particle size distribution, and inconsistent batch-to-batch quality. Microfluidic technology has emerged as a transformative tool to overcome these challenges, enabling precise, scalable, and reproducible synthesis of PLGA-based nanocarriers.
Why PLGA?
PLGA, a copolymer of lactic acid and glycolic acid, degrades into non-toxic metabolites and allows tunable release profiles by varying the monomer ratio. These characteristics make it highly suitable for encapsulating a wide range of therapeutic agents, including small molecules, proteins, peptides, and nucleic acids. However, to harness its full potential, formulation precision is critical—this is where microfluidics steps in.
What Is Microfluidics?
Microfluidics is the science and technology of manipulating tiny amounts of fluids, typically in the microliter or nanoliter range, within micro-scale channels fabricated from materials such as PDMS (polydimethylsiloxane), polyimide, aluminum, or glass capillaries.
These microchannels allow unparalleled control over mass and heat transfer, reaction kinetics, and mixing phenomena. Compared to conventional synthesis methods, microfluidic-assisted formulation excels in:
- Precise control over particle size and distribution
- Reduced reagent consumption
- Minimized batch-to-batch variability
- High reproducibility and scalability
- Integration with downstream analytical systems
Microfluidic Modalities for PLGA DDS Synthesis
Microfluidic systems for nanoparticle fabrication can be broadly classified into two modes:
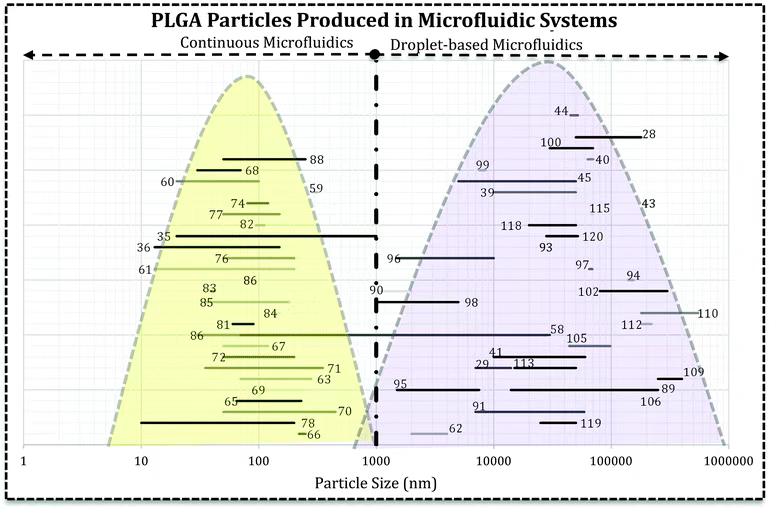
Fig. 1 Microfluidic systems for PLGA drug delivery systems.1,2
- Droplet-Based Microfluidics
Droplet microfluidics involves the formation of discrete liquid droplets via immiscible fluids. These droplets serve as tiny reaction compartments for the formation of emulsions, microparticles, and nanoparticles (NPs).
Key parameters that govern droplet formation include:
- Channel geometry: Parallel, cross-flow, and flow-focusing configurations determine droplet size and generation frequency.
- Fluid properties: Viscosity, interfacial tension, and the presence of surfactants influence the breakup of the dispersed phase.
- Flow rates: The flow rate ratio (FRR) between the dispersed and continuous phases shifts the droplet formation mode between dripping, jetting, and squeezing regimes.
In particular:
- Dripping: Occurs at low flow rates; generates monodisperse droplets with tight size distribution.
- Jetting: Higher flow velocities yield polydisperse droplets with higher surface area-to-volume ratios.
- Squeezing: Characterized by droplet deformation and breakup due to pressure buildup in confined channels.
Both active methods (involving external forces like magnetic or electric fields) and passive methods (relying on fluid flow and geometry) are used for droplet control and mixing.
- Continuous-Flow Microfluidics
In contrast to droplet systems, continuous-flow microfluidics involves the co-flow of miscible or immiscible fluids along microchannels without phase breakup. This configuration allows rapid and uniform mixing, making it ideal for nanoprecipitation and emulsification-based synthesis.
Advantages of continuous flow systems include:
- Narrower particle size distributions
- Efficient heat and mass transfer
- Minimized wall fouling and clogging
- Scalability through parallelization
Microfluidic mixing enables the fast and controlled precipitation of PLGA particles, often leading to higher encapsulation efficiency and reduced burst release.
Microfluidic Devices for PLGA-Based DDS Fabrication
Over the past two decades, a wide variety of microfluidic devices have been employed to generate PLGA nanoparticles (NPs), microparticles (MPs), and even microfibers:
- PDMS: Chip-based NP synthesis, flexible design, ease of fabrication.
- Glass capillaries: Emulsion-based MP production, optical transparency, chemical resistance.
- Phenolic resin: Drug carrier platforms, high stability, and pressure tolerance.
- Aluminum: High-throughput microreactors, heat transfer efficiency.
- Silicon wafers: Integrated Lab-on-a-Chip, CMOS-compatible, miniaturization potential.
While PLGA MPs and NPs have shown excellent performance in encapsulating hydrophilic and hydrophobic drugs, microfibers fabricated by microfluidics are less common in DDS due to drawbacks such as voids and undesirable hydrogel-like properties post-curing.
Challenges & Opportunities
Despite its advantages, microfluidic-assisted PLGA nanoparticle synthesis still faces several hurdles:
- Low throughput: Single-channel designs limit scalability for industrial use.
- Device cost: Microfabrication and maintenance expenses may be high.
- Process optimization: Different payloads and release requirements necessitate case-by-case parameter tuning.
However, emerging solutions like multi-channel parallelization, automated parameter screening, and AI-driven process control are paving the way for industrial-scale adoption.
Creative Biolabs’ Microfluidic Platform: Your Partner in DDS Innovation
At Creative Biolabs, we combine microfluidic engineering with pharmaceutical expertise to deliver customized PLGA-based drug delivery solutions. Our services include:
- Microfluidic Development Services for Drug Delivery
- Polymer Nanoparticle Synthesis Service
- Polyacrylamide Particle Synthesis Service
Creative Biolabs is known for its professionalism, scientific research, innovation, and excellent service in the laboratory. With microfluidics as the main core technology, the company continues to grow and develop. Among the focuses are precise analytical techniques and quality laboratory products and services.
References
- Rezvantalab, Sima, and Mostafa Keshavarz Moraveji. “Microfluidic assisted synthesis of PLGA drug delivery systems.” RSC advances4 (2019): 2055-2072. https://doi.org/10.1039/C8RA08972H
- Distributed under Open Access license CC BY 3.0, without modification.