Myocardial infarction (MI) is a life-threatening condition caused by the sudden blockage of coronary arteries, often accompanied by serious consequences such as abnormal electrical signal conduction, ventricular remodeling, and fibrosis. Although traditional methods such as coronary artery interventions are widely used in clinical practice, the limited regenerative capacity of myocardial tissue reduces the effectiveness of treatment. Currently, engineered hydrogel patches have been widely used as mechanical support biomaterials, especially with the introduction of conductive components aimed at restoring impaired electrical signal conduction after infarction. However, myocardial tissue has an aligned structure and exhibits centripetal synchronous contraction characteristics. Although a range of conductive hydrogels applied to myocardial tissue engineering has been widely reported, the development of anisotropic patches with long-range ordered electrical conduction that can guide the directional growth of cardiomyocytes remains a challenge.

Recently, scientists have proposed a multifunctional anisotropic cardiac patch based on microfluidic manipulation technology. The anisotropic alginate-methacrylated gelatin patch can be prepared quickly and easily through processes such as microfluidic focusing, ionic-photocrosslinking, and parallel stacking. This mild, simple, and efficient preparation strategy for anisotropic myocardial patches avoids complex manufacturing processes and strong magnetic field stimulation, and is compatible with the loading of macromolecular biopharmaceuticals (Figure 1). In vitro, this patch can mimic the anisotropy of myocardial tissue and guide the directional growth of cardiomyocytes, while also demonstrating good recovery effects in a rat myocardial infarction model. This work provides a new universal method for the design of anisotropic patches in cardiac tissue engineering and offers a new paradigm for using microfluidic technology to address challenges in tissue engineering.
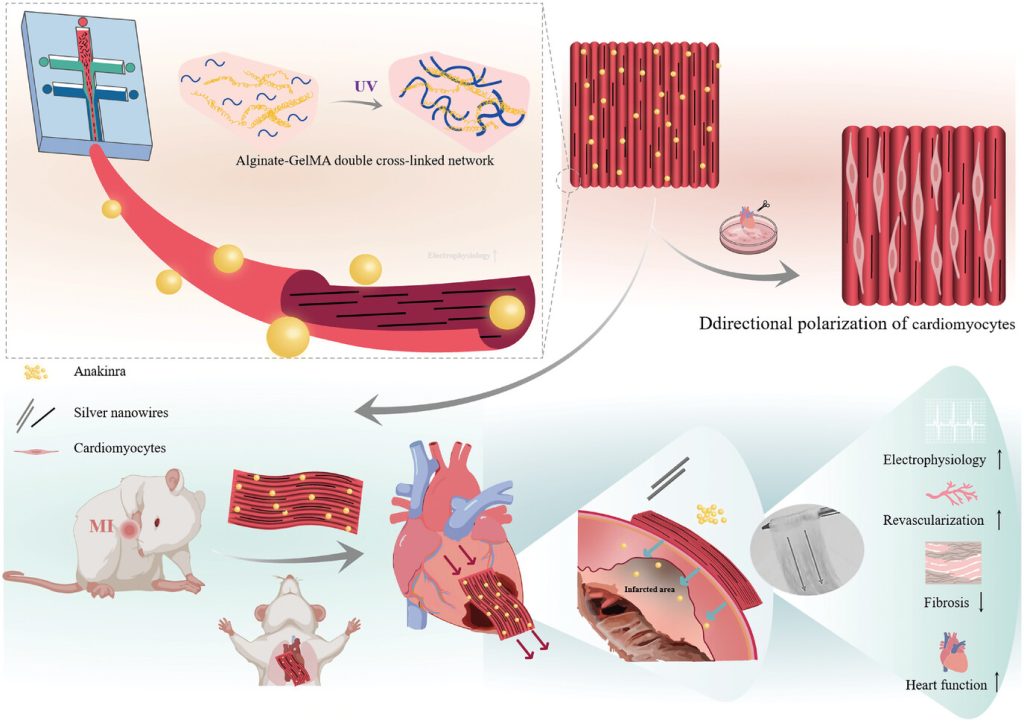 Figure 1. Preparation of anisotropic myocardial patches based on microfluidic technology and their application in myocardial infarction treatment.
Figure 1. Preparation of anisotropic myocardial patches based on microfluidic technology and their application in myocardial infarction treatment.
First, the PDMS chip design incorporates two continuous intersecting channels, with the central channel containing alginate, methacrylated gelatin (GelMA), silver nanowires, and a photoinitiator. When fluid is confined within the microscale chip, the shear alignment effect may induce the ordering of micron- and nanoscale components inside the microfluidic stream. The design of the microfluidic chip considers the construction of dual fluid focusing forces to maximize the ordered alignment of the conductive nanowires. Considering the chemical properties of the gel components, D-PBS without calcium and a low concentration of CaCl2 are perfused into the two side channels. The diffusion of Ca2+ ions triggers a crosslinking reaction with sodium alginate, further “freezing” the alignment of the silver nanowires within the gel (Figure 2).
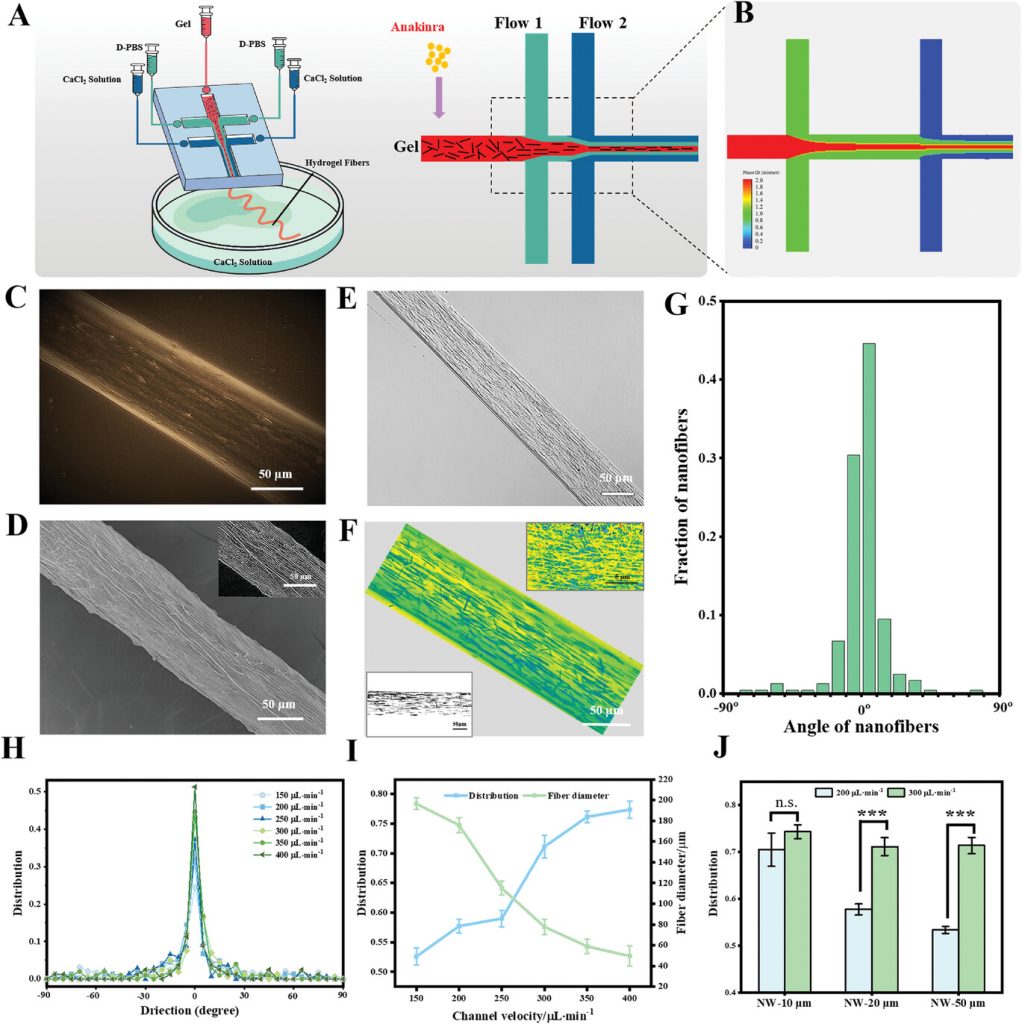 Figure 2. Preparation of anisotropic gel fibers based on microfluidic control and characterization of the directional alignment of internal nanowires.
Figure 2. Preparation of anisotropic gel fibers based on microfluidic control and characterization of the directional alignment of internal nanowires.
The anisotropic scaffold can promote directional electrical coupling between cardiomyocytes and guide them to form elongated and aligned morphologies. To study the effects of anisotropic structures on the morphology and function of cardiomyocytes, primary cardiomyocytes were extracted from neonatal rats and seeded onto different scaffolds. The results showed that patches with dual geometric and conductive anisotropy effectively induced the polarization and aligned growth of cardiomyocytes (Figure 3).
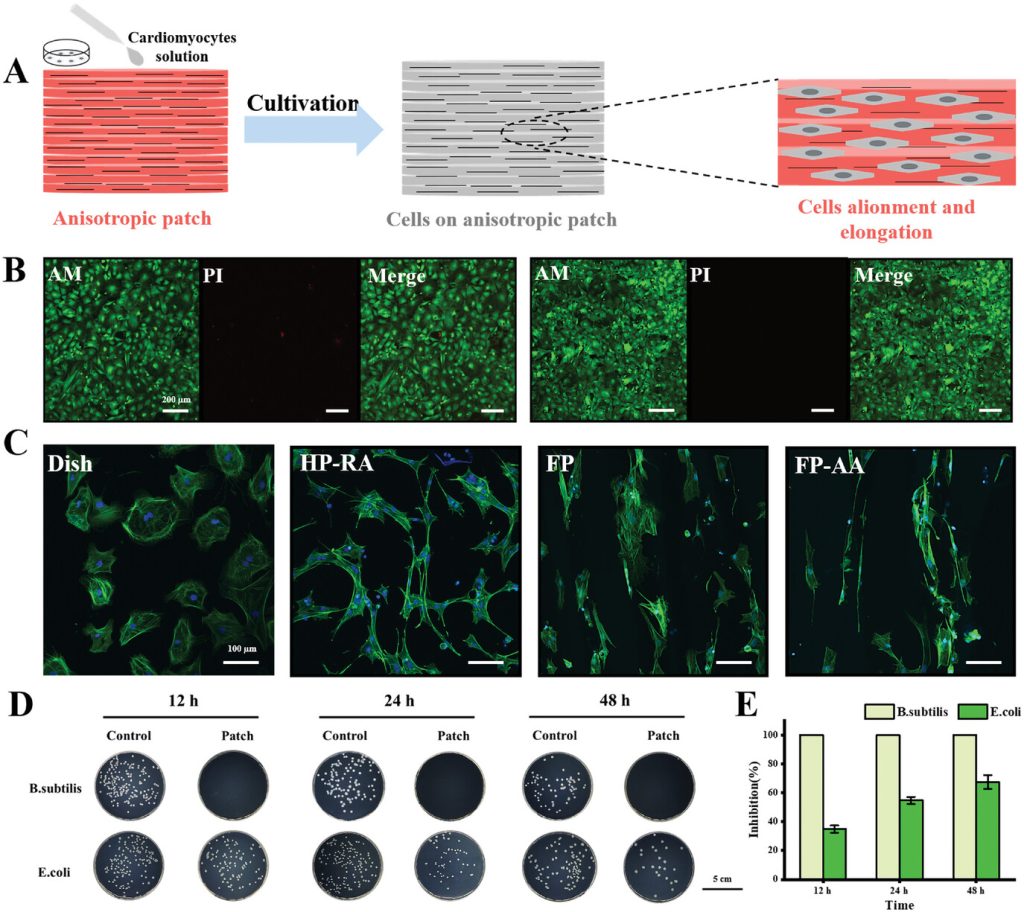 Figure 3. In vitro induction of directional cardiomyocyte growth by the anisotropic myocardial patch and characterization of its antibacterial properties.
Figure 3. In vitro induction of directional cardiomyocyte growth by the anisotropic myocardial patch and characterization of its antibacterial properties.
The left anterior descending artery of rat hearts was ligated to simulate acute myocardial infarction caused by cardiovascular occlusion, establishing a rat model of acute myocardial infarction to evaluate the cardioprotective effects of the various scaffolds. Animal experiment results indicated that after treatment with anisotropic myocardial patches, the cardiac function indicators of the rats were restored, and vascular reconstruction in the infarcted area, along with inflammation in vivo, was significantly improved (Figure 4). Further electrocardiographic monitoring and cardiac perfusion electrophysiology characterization in rats confirmed the effectiveness of the anisotropic patches in restoring electrical conduction after myocardial infarction (Figure 5).
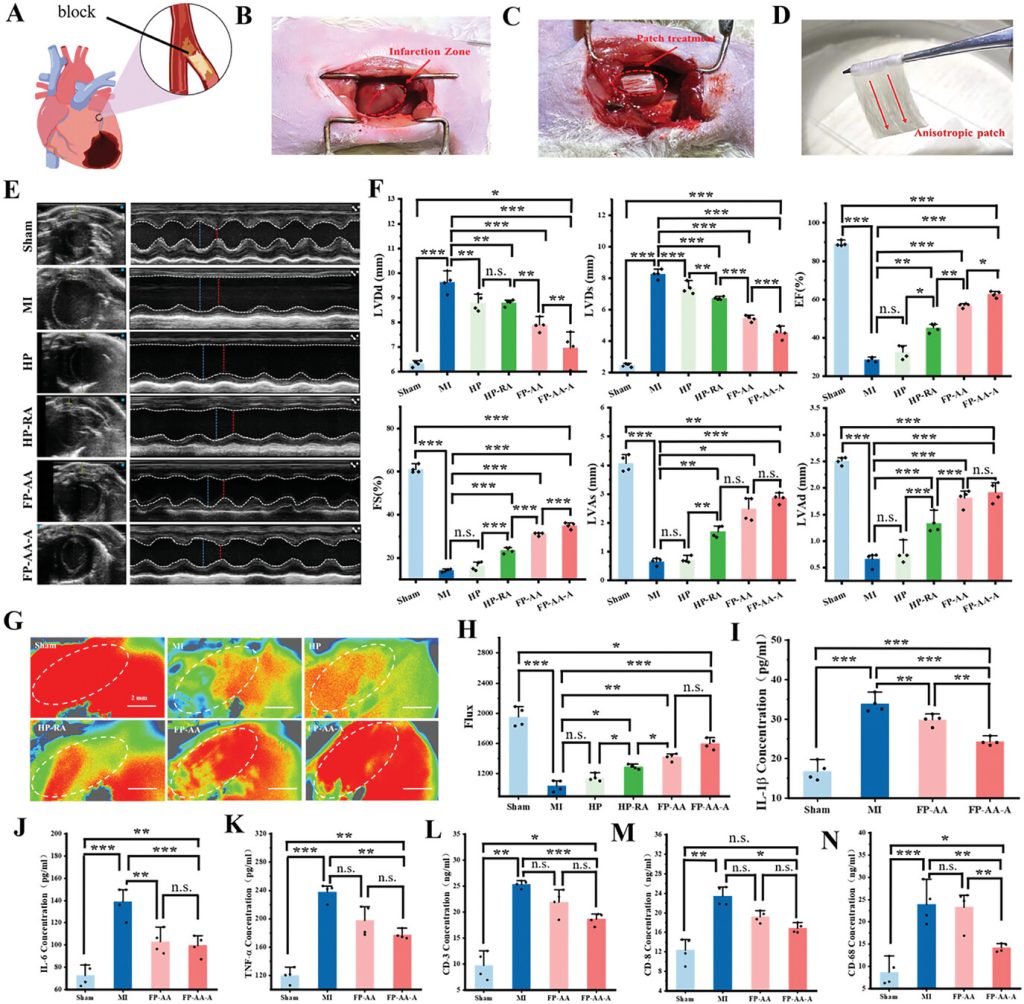 Figure 4. Therapeutic effects of the anisotropic myocardial patch in the rat myocardial infarction model.
Figure 4. Therapeutic effects of the anisotropic myocardial patch in the rat myocardial infarction model.
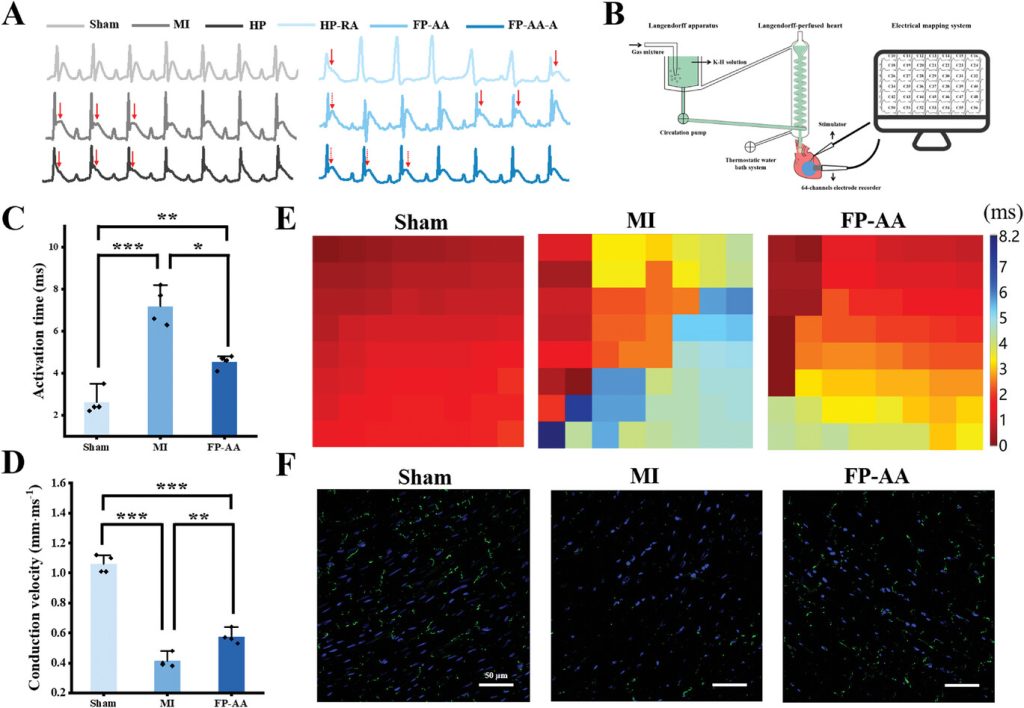 Figure 5. Electrocardiographic and electrophysiological characterization of the heart after Langendorff heart perfusion.
Figure 5. Electrocardiographic and electrophysiological characterization of the heart after Langendorff heart perfusion.
Finally, histological evaluation of heart sections from the infarcted region showed that rats treated with anisotropic patches exhibited significantly reduced fibrosis and myocardial apoptosis in the infarcted area. The treatment also inhibited left ventricular remodeling and inflammatory cell infiltration while promoting some degree of vascular regeneration in the infarcted region (Figure 6). The improvement in therapeutic outcomes for myocardial infarction may be directly attributed to the dual geometric-conductive anisotropy of the patch and the loading of anti-inflammatory active drugs.
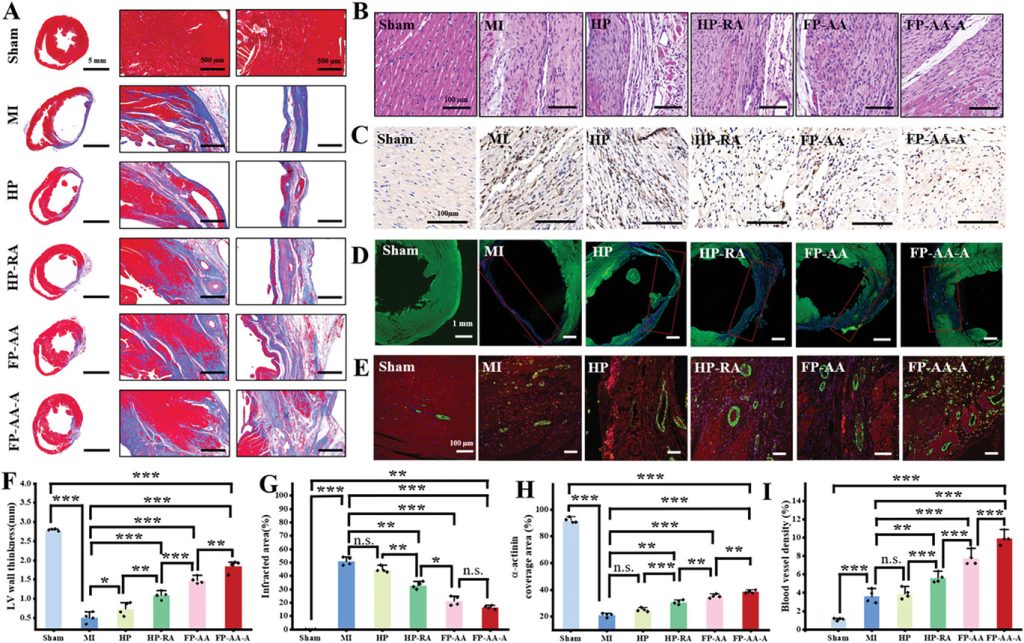 Figure 6. Staining of tissue sections in the infarcted heart region.
Figure 6. Staining of tissue sections in the infarcted heart region.
The above research findings were recently published in Advanced Materials under the title “A Multifunctional Anisotropic Patch Manufactured by Microfluidic Manipulation for the Repair of Infarcted Myocardium.”
Reference:
Jia, Xiaomeng et al. “A Multifunctional Anisotropic Patch Manufactured by Microfluidic Manipulation for the Repair of Infarcted Myocardium.” Advanced materials (Deerfield Beach, Fla.), e2404071. 16 Sep. 2024, doi:10.1002/adma.202404071
Related Services:
One-Stop Microfluidic Solutions