Extracellular vesicles (EVs) hold tremendous potential as liquid biopsy biomarkers for cancer diagnosis. Ovarian cancer (OvCa), with its high mortality rate and lack of effective early diagnostic methods, poses a severe threat to women’s health. Evidence suggests that nanoscale small extracellular vesicles (sEVs) carrying cell-specific components derived from OvCa can serve as potential diagnostic biomarkers. Therefore, developing a liquid biopsy technology capable of rapidly, efficiently, and sensitively detecting nanoscale sEVs is of great significance for achieving the early and accurate diagnosis of ovarian cancer.
Recently, scientists reported a study titled “Sensitive phenotyping of serum extracellular vesicles on a SERS-microfluidic platform for early-stage clinical diagnosis of ovarian carcinoma” in the Elsevier journal Biosensors and Bioelectronics. This research introduced a novel method, S-MMEV (SERS-Microfluidic Multiplexed EV detection), which utilizes a surface-enhanced Raman scattering (SERS)-based multichannel microfluidic chip to study the surface protein characteristics of small extracellular vesicles (sEVs). By analyzing sEV phenotypes, the study successfully distinguished healthy individuals from ovarian cancer patients, enabling precise diagnosis of early-stage ovarian cancer. This innovative approach provides a valuable new method for cancer early diagnosis based on sEVs, with significant potential for clinical application.
The detection mechanism of S-MMEV is illustrated in Figure 1. Small extracellular vesicles (sEVs) derived from ovarian cancer are introduced into a microfluidic chip equipped with seven parallel microchannels, where they are identified using SERS probes conjugated with different specific antibodies. These SERS probes are prepared by conjugating Au@Ag nanoparticles with the Raman reporter molecule MGITC and antibodies targeting sEV surface biomarkers. The targeted biomarkers include general EV markers (tetraspanins: CD9, CD81) and ovarian cancer tumor markers (EpCAM, CD24, CA125, EGFR). By detecting the signal intensity of the SERS nanoprobes in each microchannel, the expression levels of different sEV surface biomarkers are revealed. The signal intensities of the six biomarkers provide a visual representation of the phenotypic variations in sEVs. This enables the phenotypic profiling of sEVs from both cell culture media and clinical samples (with IgG as a control), enabling accurate differentiation between ovarian cancer patients, including those in early stages (I-II), and healthy controls.
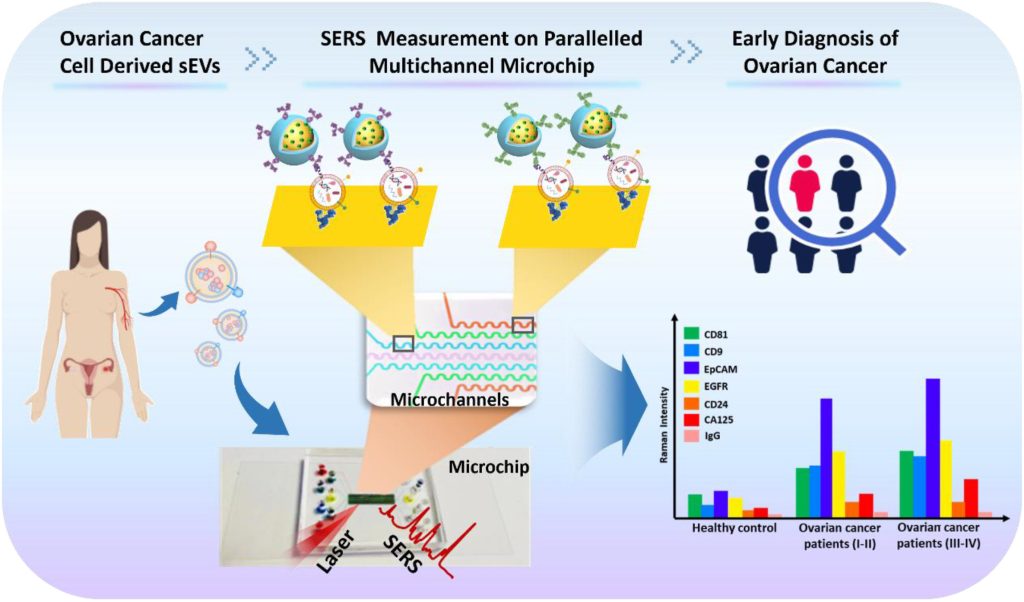 Figure 1. Schematic illustration of S-MMEV assay mechanism for investigating phenotypic changes in sEVs for clinical diagnosis of ovarian cancer
Figure 1. Schematic illustration of S-MMEV assay mechanism for investigating phenotypic changes in sEVs for clinical diagnosis of ovarian cancer
As shown in Figure 2, the microfluidic chip adopts a dual-layer design, featuring a PDMS layer with seven parallel microchannels and a gold nanofilm layer on a glass slide. This configuration enables the parallel detection of multiple biomarkers, thereby enhancing detection accuracy. Reservoirs are located att the inlets of the seven microchannels, and liquid is introduced via a multichannel microfluidic pump that pulls from the outlets. Within the microchannels, capture antibodies (anti-CD63) are first introduced for surface modification, followed by the introduction of sEVs of the same batch, concentration, and volume. After blocking unreacted surfaces with BSA, SERS nanoprobes are introduced for biomarker recognition. The experimental setup also validated the effectiveness of the modification method using fluorescently labeled antibodies.
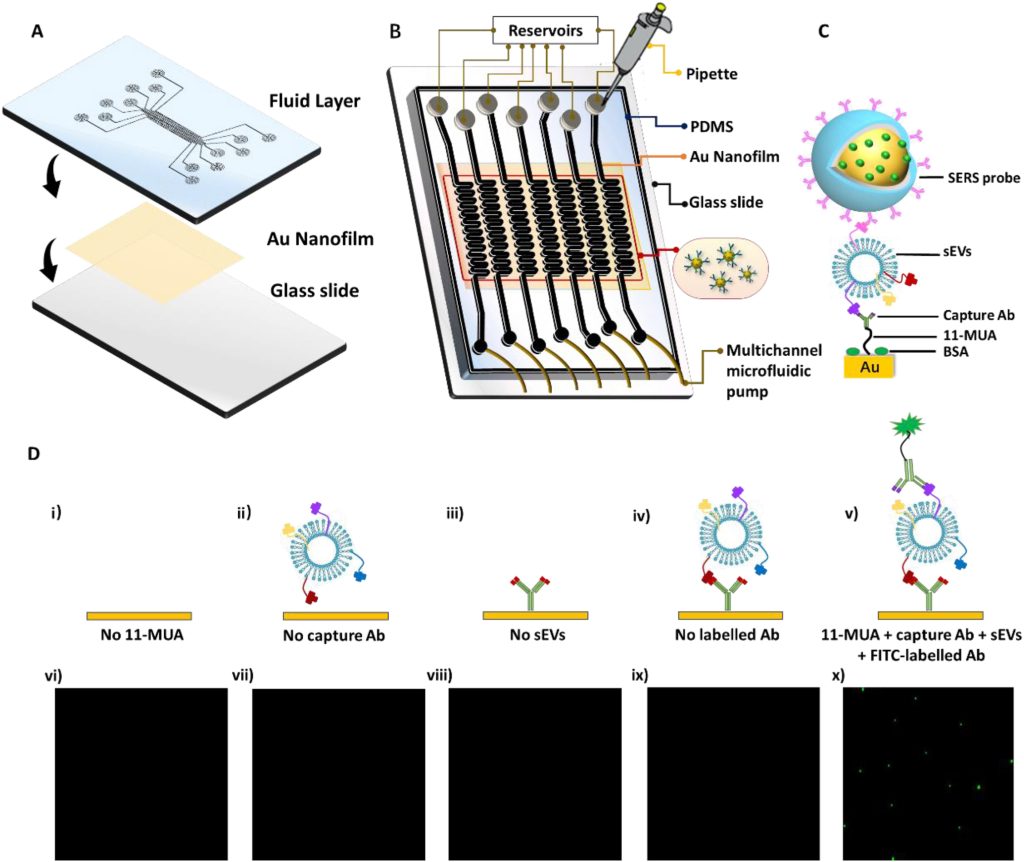 Figure 2. Microfluidic chip design, structure, surface modification, and characterization
Figure 2. Microfluidic chip design, structure, surface modification, and characterization
The research team successfully extracted sEVs from ovarian granulosa cells (HO23), ovarian cancer cells (OVCAR3), and serum, and performed characterization (Figure 3). TEM results revealed the typical cup-shaped structure of exosomes, NTA results confirmed that the size of sEVs ranged from 100 to 200 nm, and WB characterization showed that sEVs from all three sources expressed the general EV protein CD63 and ovarian cancer tumor marker proteins such as EpCAM and EGFR.
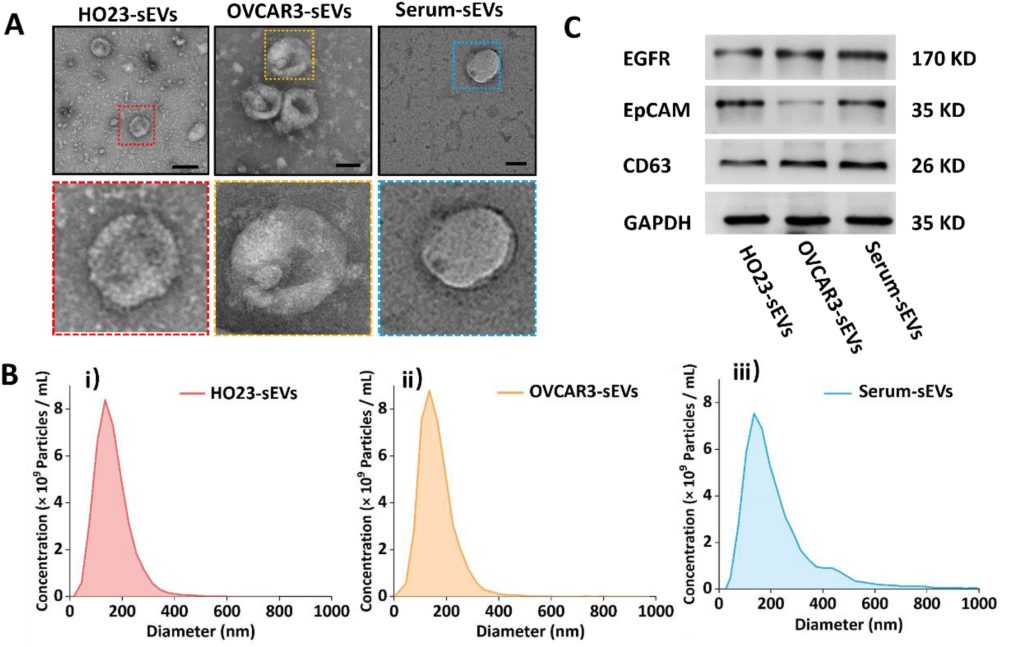 Figure 3. TEM, NTA, and WB characterization of sEVs derived from HO23, OVCAR3, and serum
Figure 3. TEM, NTA, and WB characterization of sEVs derived from HO23, OVCAR3, and serum
The research team evaluated the stability and detection sensitivity of the SERS probes used in the S-MMEV method. Experimental results demonstrated excellent sensitivity and specificity, effectively distinguishing sEVs derived from normal and ovarian cancer cell culture media.
The team further applied this technology to clinical sample testing to validate its application in early diagnosis. The study included 36 clinical samples, consisting of 10 healthy controls (HC), 15 early-stage OvCa patients (I-II), 7 late-stage OvCa patients (III-IV), and 4 post-operative OvCa patients.
The heatmap results from S-MMEV detection of sEVs in these clinical samples (Figure 3A) revealed varying abundances of six sEV biomarkers among the samples. Violin plot analysis (Figure 3B) demonstrated statistically significant differences (P < 0.0001) in detection values between HC and OvCaP (I-II) for individual sEV biomarkers. Significant differences (P < 0.0001) were also observed between HC and OvCaP (III-IV) or OvCaP AO for tumor biomarkers EpCAM, CD24, and CA125 (Figure 3B iii, v, vi), while EGFR showed relatively high differences (P < 0.001).
Further receiver operating characteristic (ROC) analysis was conducted to evaluate the sensitivity, specificity, and area under the curve (AUC) for the S-MMEV method in detecting both individual and combined biomarkers to distinguish healthy controls (HC) from cancer patients (Figure 3C). The results showed that AUC values for individual biomarkers (Figure 3C i-vi) were lower than those obtained when combining general EV proteins with tumor-specific biomarkers. When all six biomarkers were used together, AUC values exceeded 0.94 for distinguishing HC from OvCa (I-II) and HC from OvCa (III-IV and AO). Notably, for HC versus OvCa (I-II), the sensitivity reached 1.00, accuracy was 0.95, and the AUC value was 0.9467.
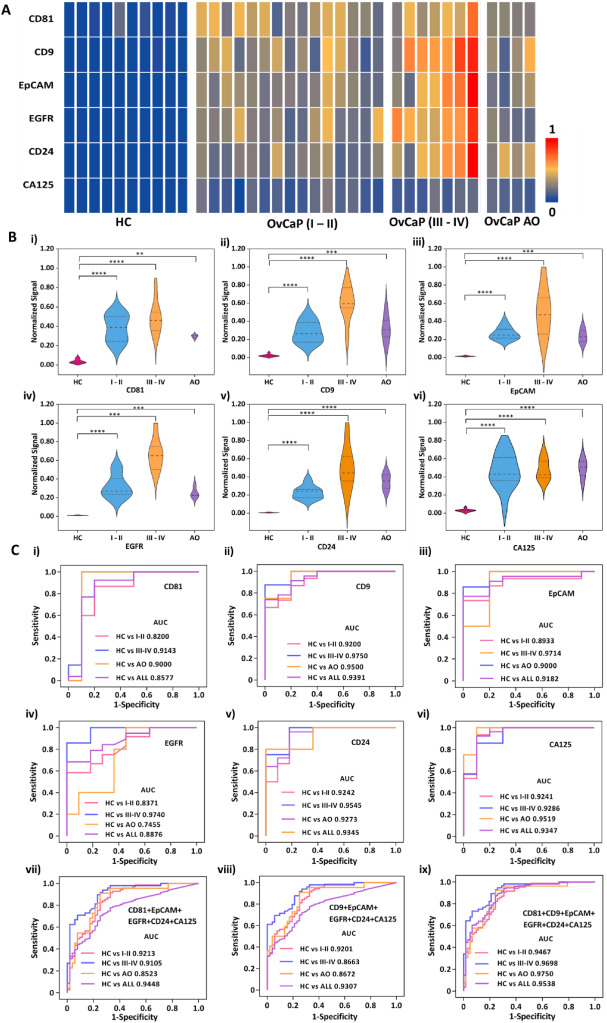 Figure 4. Molecular profiling analysis of protein biomarkers in OvCa
Figure 4. Molecular profiling analysis of protein biomarkers in OvCa
In summary, this study developed a SERS-microfluidic chip for sEV detection (S-MMEV) to investigate phenotypic variations in sEVs. By analyzing these phenotypes, the method successfully distinguished healthy individuals from OvCa patients and further identified early-stage OvCa cases. With high sensitivity and specificity, the parallel channel design effectively avoided interference among different phenotypic biomarkers, providing an ideal tool for early ovarian cancer diagnosis. Compared to traditional detection methods, the S-MMEV strategy offers higher accuracy and sensitivity in clinical sample diagnosis with reduced sample consumption. Moreover, this method is not limited to ovarian cancer detection; it can be adapted for the early and precise diagnosis of other tumors by designing SERS probes targeting different tumor biomarkers.
Reference:
Chen, Xingya et al. “Sensitive phenotyping of serum extracellular vesicles on a SERS-microfluidic platform for early-stage clinical diagnosis of ovarian carcinoma.” Biosensors & Bioelectronics vol. 267 (2025): 116724. doi:10.1016/j.bios.2024.116724
Related Services: