Exosomes are nanoscale extracellular vesicles that carry a variety of biomolecules and play an important role in physiological and pathological processes. Their characteristics make them ideal biomarkers for cancer diagnosis. Studies have shown that exosomes carry tumor-specific information and can affect the tumor microenvironment and metastasis. For example, exosomes secreted by breast cancer cells overexpress the HER2 protein and promote tumor proliferation. However, traditional exosome detection methods such as mass spectrometry, enzyme-linked immunosorbent assay (ELISA), and flow cytometry face challenges, including complex operations, lengthy processing times, and the need for precision equipment, which limits their clinical applications. To this end, researchers have been seeking faster and more sensitive detection technologies.
In the past decade, a variety of biosensing technologies such as fluorescence, colorimetry, electrochemistry, and field-effect transistors (FETs) have been used for exosome detection. Among these, surface-enhanced Raman scattering (SERS) technology stands out due to its high sensitivity and unique fingerprinting capabilities. Compared to fluorescence, SERS has the advantages of small photobleaching, a wide excitation wavelength range, and the ability to conduct multiple detections. Despite its broad potential, SERS technology still faces challenges in portability and simplified sample preparation. Therefore, the development of a user-friendly SERS platform with simple sample preparation is of great significance to promote the clinical application of exosome detection.

Recently, scientists have developed a new cancer exosome detection platform based on SERS and droplet microfluidic technology. This platform allows for the rapid, highly sensitive and high-throughput detection of HER2-positive exosomes secreted by breast cancer cells, providing a new technical means for early diagnosis of cancer. The relevant results were published in the authoritative biosensor journal ACS Sensors under the title “SERS-Based Droplet Microfluidic Platform for Sensitive and High-Throughput Detection of Cancer Exosomes”.
Research Content:
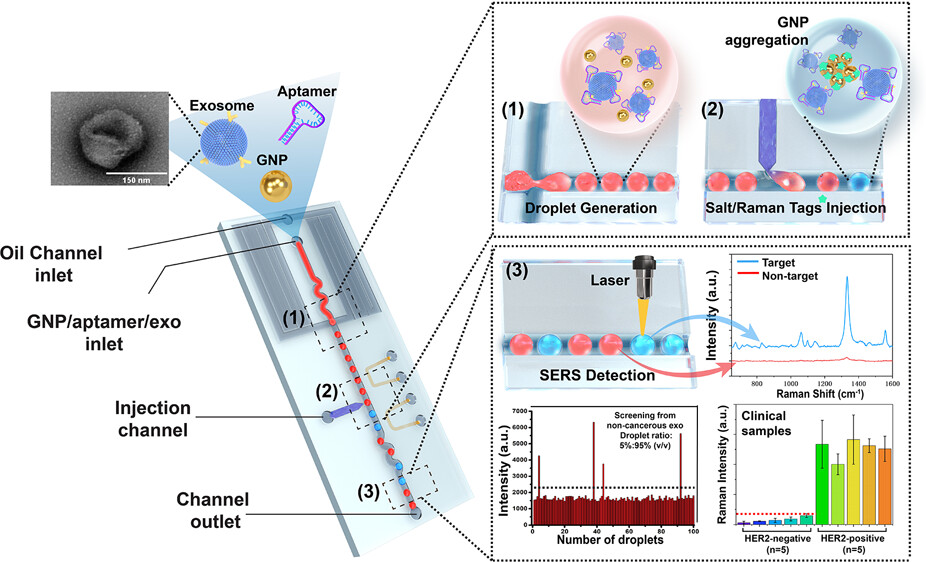
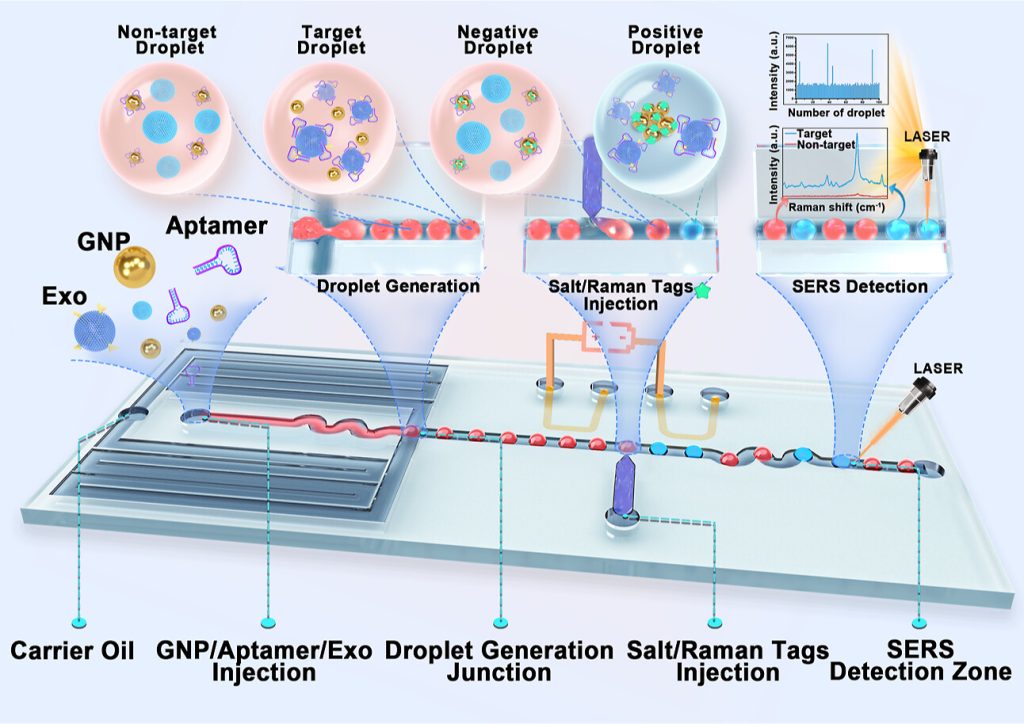 Figure 1. Illustration of the process of the SERS-based droplet microfluidic platform for detecting HER2-positive exosomes.
Figure 1. Illustration of the process of the SERS-based droplet microfluidic platform for detecting HER2-positive exosomes.
The droplet-based microfluidic platform integrated with the SERS aptamer sensor consists of four main parts: the droplet generator, salt/Raman tag microinjection unit, mixing part, and SERS signal observation part (Figure 1). Its working principle is as follows:
1. Droplet generation: A mixture of gold nanoparticles (GNPs), exosomes (Exo), and HER2 aptamers is injected into the sample inlet and encapsulated into single droplets at the droplet generation junction.
2. Aptamer-exosome binding: In the target droplets containing HER2-positive exosomes, the HER2 aptamer specifically binds to the HER2 biomarker on the surface of the exosomes, causing the GNPs to lose their protective coating. In contrast, in the non-target droplets, the HER2 aptamer adsorbes onto the surface of the GNPs to form a protective layer.
3. Salt/Raman tag injection: The salt/Raman tag solution is injected into each droplet through the microelectrode injection unit of the microinjection part.
4. Salt-induced GNP aggregation: In the target droplets containing HER2-positive exosomes, the injected salt solution induces aggregation of the unprotected GNPs with the Raman tags, generating a hotspot-enhanced SERS signal. In the non-target droplets, the GNPs remain unaggregated due to electrostatic repulsion ensured by the protective aptamer layer.
5. SERS signal detection: When the droplets pass through the SERS detection zone under a 785 nm laser, the SERS signal from each droplet is measured. By analyzing the signal intensity, researchers can identify the target droplets containing HER2-positive exosomes.
The researchers used the salt-induced gold nanoparticle aggregation process on the chip to produce an enhanced SERS signal in the presence of HER2-positive exosomes. This method not only simplifies the sample preparation process, but also greatly improves detection sensitivity, enabling the detection of HER2-positive exosomes at a concentrations as low as 4.5 log10 particles/mL within just 5 minutes. The high-throughput capability of this microfluidic chip can quickly screen hundreds of droplets per minute, making it suitable for large-scale clinical diagnosis. More importantly, the platform has been successfully applied to clinical sample testing and can effectively distinguish between HER2-positive and HER2-negative breast cancer patients.
This achievement provides new ideas for the development of early cancer diagnosis technologies based on liquid biopsy.
Reference:
Ho, Kwun Hei Willis et al. “SERS-Based Droplet Microfluidic Platform for Sensitive and High-Throughput Detection of Cancer Exosomes.” ACS sensors vol. 9,9 (2024): 4860-4869. doi:10.1021/acssensors.4c01357
Related Services:
Microfluidics-Based Analysis in Exosome Isolation