Reactive sulfur species (RSS) and reactive oxygen species (ROS) are closely related to the physiological and pathological processes of redox reactions and oxidative stress. Hydrogen sulfide (H₂S), a reactive sulfur species, has been linked to a variety of diseases including cancer, inflammation, hypertension, and neurodegenerative diseases. Hydrogen peroxide (H₂O₂), a reactive oxygen species, is often detected at abnormal levels in cardiovascular disease, inflammation, cancer, diabetes, and liver and kidney diseases. Notably, H₂O₂ can significantly enhance cancer therapy through its interaction with H₂S. Current research shows that H₂O₂ promotes the H₂S signaling pathway under oxidative stress conditions. Therefore, there is an increasing demand for real-time and in situ monitoring of H₂S and H₂O₂ interactions in biological samples to elucidate the mechanisms of disease development from the perspective of redox signaling.
Recently, researchers have proposed a fully integrated electrochemical sensor based on microfluidics for real-time dynamic monitoring of H₂S and H₂O₂ secreted by various biological samples. A recent study, published in the journal Biosensors and Bioelectronics under the title “Bimetal−organic framework-integrated electrochemical sensor for on-chip detection of H₂S and H₂O₂ in cancer tissues,” describes this innovative approach. The research team clinically validated the RuCu-HHTP sensing platform by locally detecting the secretion of H₂S and H₂O₂ in human colorectal cancer (CRC) cells and tissues. They also evaluated the chemotherapy sensitivity of these cancer tissues, which aids in the early diagnosis of diseases related to H₂S and H₂O₂, the assessment of tumor progression and prognosis, and ultimately improves the predictive ability of precision medicine.
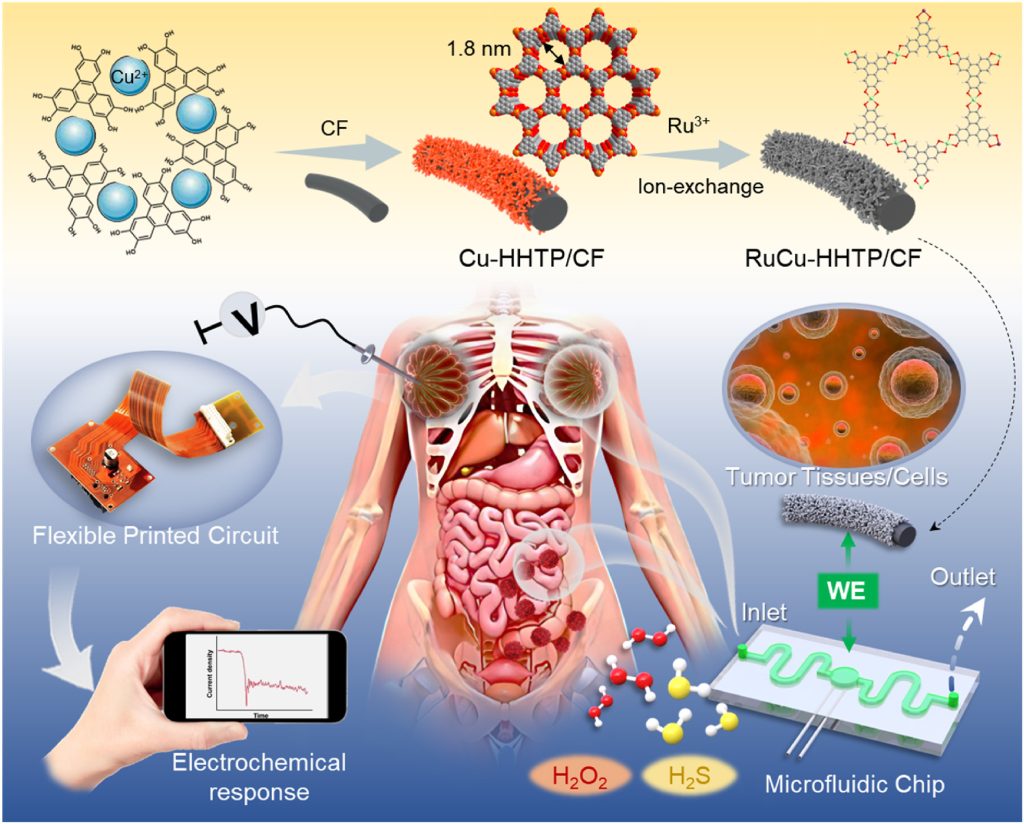 Figure 1. Schematic diagram of the preparation process of the RuCu-HHTP/GF microelectrode and the electrochemical sensor integrated multi-channel microfluidic chip for detecting biomolecule-induced signals in tumor cells and tissues.
Figure 1. Schematic diagram of the preparation process of the RuCu-HHTP/GF microelectrode and the electrochemical sensor integrated multi-channel microfluidic chip for detecting biomolecule-induced signals in tumor cells and tissues.
The research team confirmed the insertion of Ru ions into the Cu-HHTP framework using inductively coupled plasma emission spectroscopy. Compared with CF, RuCu-HHTP/CF exhibits a wider pore size distribution, an open porous interconnected structure, and a larger surface area, all of which enhance molecular diffusion during electrochemical redox reactions. Cyclic voltammetry (CV) measurements and electrochemical impedance spectroscopy (EIS) tests revealed that RuCu-HHTP/CF not only has excellent reaction rates and kinetics but also demonstrates the lowest charge transfer resistance and superior electrochemical performance. At the same time, the unique microstructure of RuCu-HHTP has a pre-concentration effect, significantly enhancing electron transfer behavior in H₂S redox dynamics. Both the oxidation and reduction current signals increased significantly with H₂S concentration in a concentration-dependent manner (Figure 2A, 2B). Moreover, RuCu-HHTP/CF microelectrodes show high interference immunity to common biological and physiological substances (Figure 2C, 2D). The sensor demonstrates good reliability (Figure 2E) and excellent long-term stability (Figure 2F).
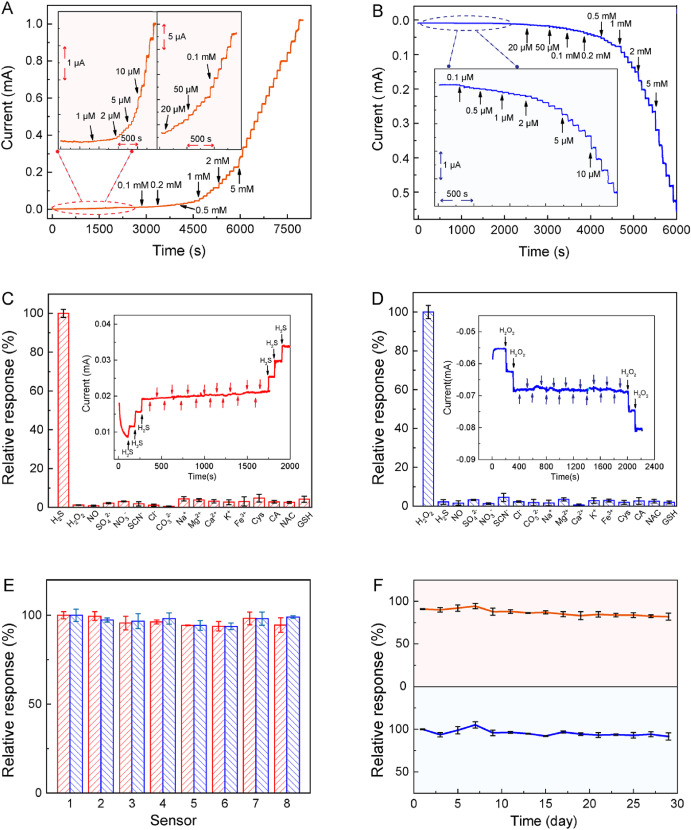 Figure 2. Current-time response of the RuCu-HHTP/CF microelectrode when different ratios of (A) H₂S and (B) H₂O₂ were injected into 0.1 M PBS; the relative response to RuCu-HHTP/CF to these interferences (1 mM) is equal to the response of the target analytes (C) H₂S and (D) H₂O₂; (E) Repeatability and (F) long-term stability of the RuCu-HHTP/CF sensor for detecting H₂S and H₂O₂.
Figure 2. Current-time response of the RuCu-HHTP/CF microelectrode when different ratios of (A) H₂S and (B) H₂O₂ were injected into 0.1 M PBS; the relative response to RuCu-HHTP/CF to these interferences (1 mM) is equal to the response of the target analytes (C) H₂S and (D) H₂O₂; (E) Repeatability and (F) long-term stability of the RuCu-HHTP/CF sensor for detecting H₂S and H₂O₂.
The electrochemical microfluidic chip, integrated with RuCu-HHTP/CF microelectrodes, consists of three layers, including a PET substrate, a microfluidic channel patterned on a PDMS substrate, and a PET cover (Figure 3A). The custom chip uses a three-electrode system, where the RuCu-HHTP/CF microelectrode serves as the WE, and the Pt and Ag/AgCl electrodes serve as the CE and RE, respectively. It connects to a reusable electrochemical workstation to establish an electrochemical microfluidic monitoring system to achieve multi-channel electrochemical measurements, signal processing, and wireless transmission (Figures 3B, 3C).
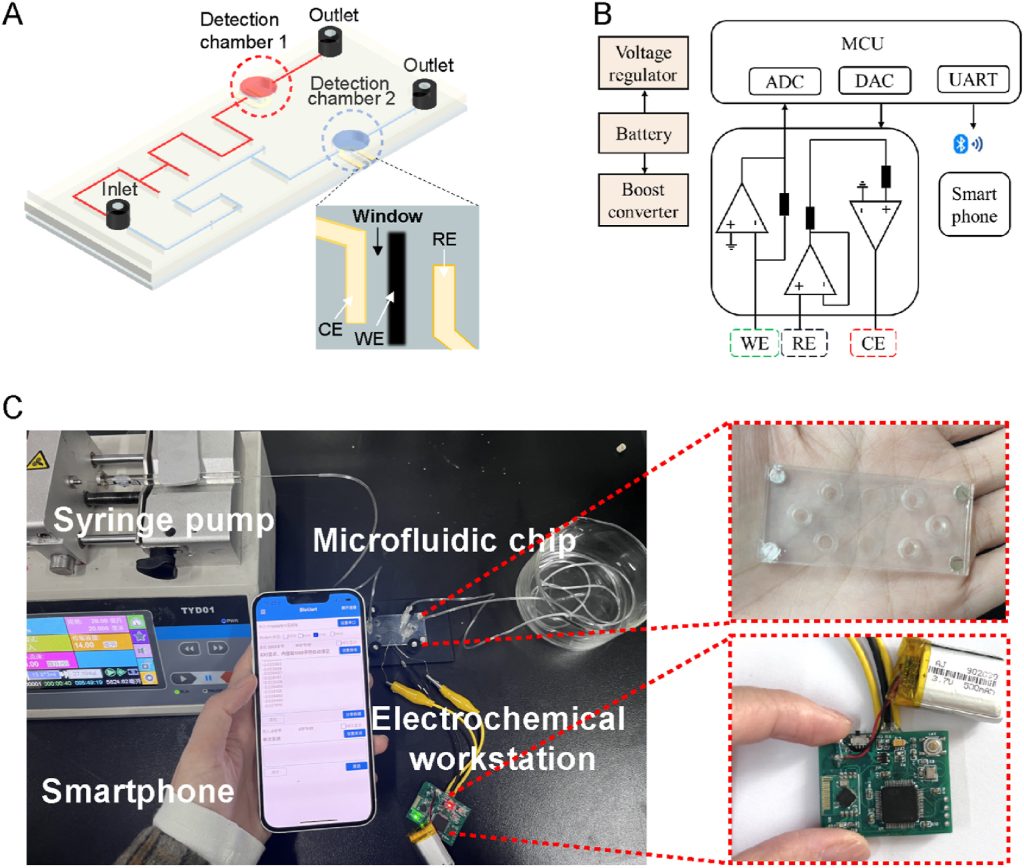 Figure 3. (A) Schematic diagram of the microfluidic device structure based on the three-electrode system; (B) System-level block diagram of the electronic system; (C) Photograph of the proposed fully integrated electrochemical microfluidic sensing system.
Figure 3. (A) Schematic diagram of the microfluidic device structure based on the three-electrode system; (B) System-level block diagram of the electronic system; (C) Photograph of the proposed fully integrated electrochemical microfluidic sensing system.
The research team used the fluorescence double staining method to evaluate the biocompatibility of RuCu-HHTP/CF. Dark field fluorescence images showed that the cells remained basically viable after 6 hours of incubation with RuCu-HHTP/CF (Figure 4A). In addition, using a standard cell counting kit, the cell viability remained above 90% after 12 hours of incubation with RuCu-HHTP/CF. These qualitative and quantitative analyses indicate that the RuCu-HHTP/CF microelectrode has good biocompatibility.
The microelectrode was placed near the cells (Figure 4B) to stimulate the release of H₂S from living cells (Figure 4C). The results confirmed that the enhanced amperometric current response originated from H₂S secreted by living cells, while the cell-free control group showed no current response (Figure 4D). Similarly, the correlation between the amperometric current response and H₂O₂ generation was observed (Figure 4E). The quantitative results obtained from multiple cells using wireless microfluidic electrochemical sensors combined with RuCu-HHTP/CF microelectrodes revealed differences in H₂S and H₂O₂ release across different types of cancer cells. Notably, tumor cells produced higher levels of H₂S and H₂O₂ compared to normal cells. This positive correlation has significant potential in cancer diagnosis and biomedical research.
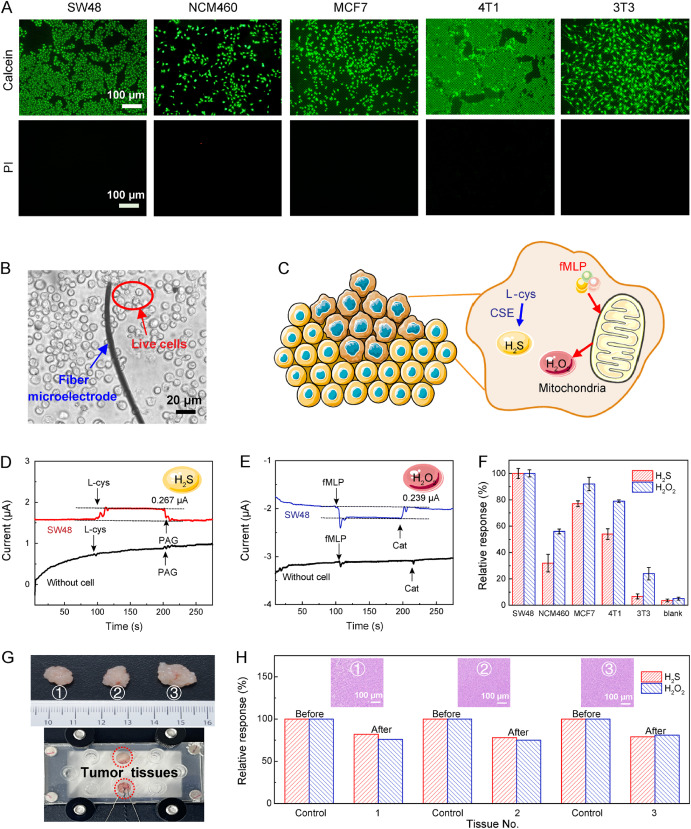 Figure 4. (A) Fluorescence images of different cells detected using calcein-AM/PI; (B) Super-resolution digital microscopy images of RuCu-HHTP/CF microelectrodes monitoring living cells; (C) Schematic diagram of stimulating living cells to secrete H₂S and H₂O₂; (D) Current response of RuCu-HHTP/CF to L-cys and PAG with and without cells added to the solution under 0.25V; (E) Current response of RuCu-HHTP/CF to fMLP and catalase with and without cells added to the solution under 0.7V; (F) Histogram of the relative increase in current associated with H₂S and H₂O₂ released by different cells; (G) Digital photos of different CRC anatomical tissues and electrochemical microfluidic sensing system embedded in tumor tissues; (H) Histogram of the relative current response of H₂S and H₂O₂ secreted by CRC tissues before and after 1 hour of chemotherapy.
Figure 4. (A) Fluorescence images of different cells detected using calcein-AM/PI; (B) Super-resolution digital microscopy images of RuCu-HHTP/CF microelectrodes monitoring living cells; (C) Schematic diagram of stimulating living cells to secrete H₂S and H₂O₂; (D) Current response of RuCu-HHTP/CF to L-cys and PAG with and without cells added to the solution under 0.25V; (E) Current response of RuCu-HHTP/CF to fMLP and catalase with and without cells added to the solution under 0.7V; (F) Histogram of the relative increase in current associated with H₂S and H₂O₂ released by different cells; (G) Digital photos of different CRC anatomical tissues and electrochemical microfluidic sensing system embedded in tumor tissues; (H) Histogram of the relative current response of H₂S and H₂O₂ secreted by CRC tissues before and after 1 hour of chemotherapy.
Reference:
Xu, Yun et al. “Bimetal-organic framework-integrated electrochemical sensor for on-chip detection of H2S and H2O2 in cancer tissues.” Biosensors & bioelectronics vol. 260 (2024): 116463. doi:10.1016/j.bios.2024.116463
Related Services:
One-Stop Microfluidic Solutions