Background
Continuous monitoring of clinically relevant biomarkers in interstitial fluid (ISF) using microneedle (MN)-based assays has the potential to transform healthcare. Researchers from McMaster University have proposed an integrated system, called the “Wearable Aptalyzer,” fabricated by combining biocompatible hydrogel MN (HMN) arrays for ISF extraction with electrochemical aptamer-based biosensors for in situ monitoring of blood analytes, for real-time and multiplexed monitoring of glucose and lactate in ISF. Validation experiments using type 1 diabetes mouse and rat models demonstrated a strong correlation between measurements collected from the ISF-based Wearable Aptalyzer and those obtained from gold standard techniques for glucose and lactate. The Wearable Aptalyzer effectively addresses the limitations inherent to enzymatic detection methods as well as solid MN biosensors and the need for reliable and multiplexed in vivo monitoring of bioanalytes. The relevant research results were published in the journal Advanced Materials under the title “Wearable Aptalyzer Integrates Microneedle and Electrochemical Sensing for In Vivo Monitoring of Glucose and Lactate in Live Animals”.

Manufacturing of Wearable Aptalyzer
The integrated Wearable Aptalyzer combines HMN patches for ISF extraction with electrodes functionalized with redox aptamers for target analysis. Briefly, hyaluronic acid (HA) was chemically modified with methacrylic anhydride (MA) overnight. The MeHA obtained by precipitation and dialysis purification was then freeze-dried. Subsequently, the HC-MeHA solution was cast into the patterned mold to fabricate the HMN patch. After an overnight drying process, the patch was demolded and cross-linked under ultraviolet (UV) irradiation for 40 min. Next, 100 μL of LC-MeHA was applied to the functionalized electrodes to form a thin adhesive layer covering the working electrode, counter electrode, and reference electrode. Among them, LC-MeHA acted as a glue to attach the HMN patch to the solid electrode. Subsequently, the cross-linked HMN patch was trimmed, placed on the LC-MeHA, and air-dried for 30 min. Finally, the integrated device was further cross-linked under UV for 10 min. LC-MeHA allowed electrodes to be firmly attached to the HMN patch, thus enhancing the robustness of the integrated device in subsequent animal experiments.
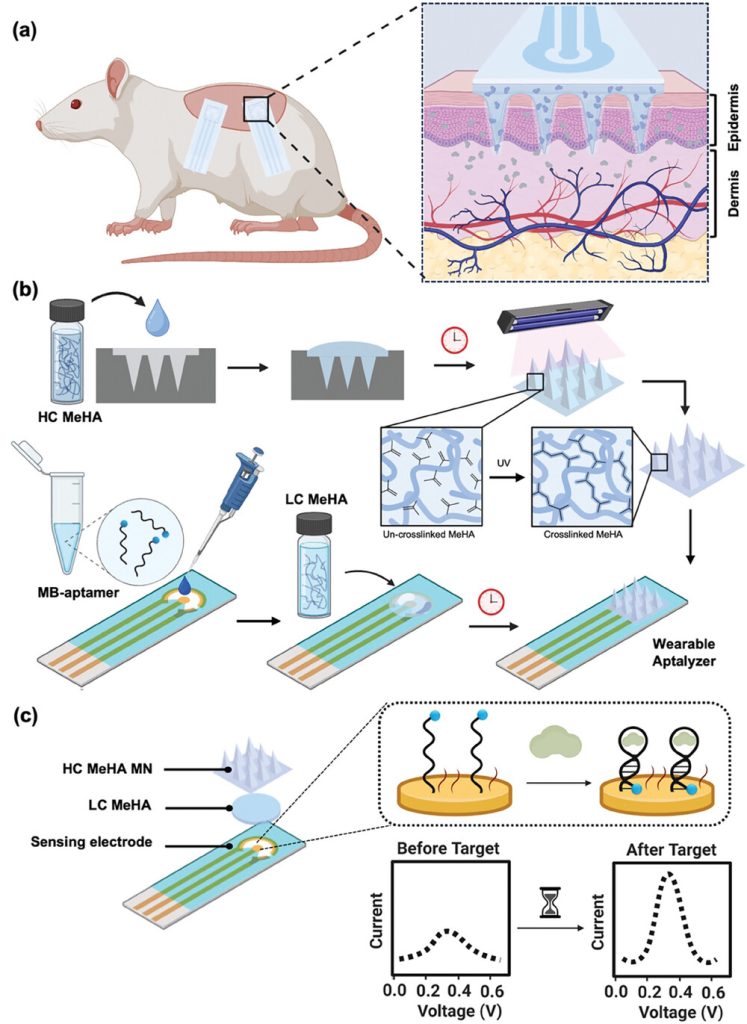 Figure 1. Fabrication of the Wearable Aptalyzer for in vivo analysis
Figure 1. Fabrication of the Wearable Aptalyzer for in vivo analysis
This study converts the integrated patch/electrode device into a wearable aptamer. Specifically, the researchers anchored a well-characterized aptamer (for lactic acid or glucose) to a gold working electrode via gold thiol chemistry and verified its adhesion using electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV). In addition, the aptamer was labeled with methylene blue as a redox reporter for electrochemical signal transduction. Upon target binding, the redox aptamer undergoes a conformational change that brings the methylene blue closer to the gold surface, increasing the electron transfer rate compared to when the target is absent.. Square wave voltammetry (SWV) was used to measure the signal generated by the Wearable Aptalyzer. The difference in charge transfer kinetics between the bound and unbound states of the redox aptamer was used to calculate the kinetic differential measurement (KDM). This method effectively reduces sensor-to-sensor variability, corrects for signal drift, and improves the signal-to-noise ratio. The signal-on and signal-off frequencies were determined using the Lovric form. The glucose and lactate sensors exhibited signal-on and signal-off responses at higher frequencies (80 Hz for glucose and 200 Hz for the lactate aptamer) and lower frequencies (5 Hz for the glucose and 15 Hz for lactate aptamer), respectively.
Physical Characterization of Wearable Aptalyzer
After fabricating the HMN patches, their physical properties were first evaluated. To ensure effective skin penetration, it is critical that the HMN patches have sharp and intact needles. The morphology of the HMN patches was evaluated using scanning electron microscopy (SEM), which showed intact and sharp needle tips. Next, the porosity of the HC-MeHA and LC-MeHA hydrogels was investigated by calculating the average pore size of the corresponding swollen hydrogels imaged using SEM. The porosity of HC-MeHA and LC-MeHA was 37% and 65%, respectively. HC-MeHA forms a dense polymer network within the HMNs, thereby maintaining its structure after water absorption. In contrast, LC-MeHA acts as an adhesion layer between the HMN patch and the electrode, forming a less dense polymer network. The extent of cross-linking depends mainly on the polymer content, the duration of UV curing, and the concentration of the cross-linking agent. To investigate the duration required for the HMN patch to collect sufficient ISF, the swelling ability of HMNs was evaluated using porcine skin. HMN patches were prepared using HC-MeHA, with a layer of LC-MeHA added to the back (HC+LC-MeHA HMN), and applied to a piece of pig skin for different durations, after which their corresponding swelling rates were measured. We observed that the HC+LC-MeHA HMN patch reached a maximum swelling of 119.45% within 6 minutes after application on pig skin, indicating that HMN can quickly and effectively collect ISF samples in a real skin environment.
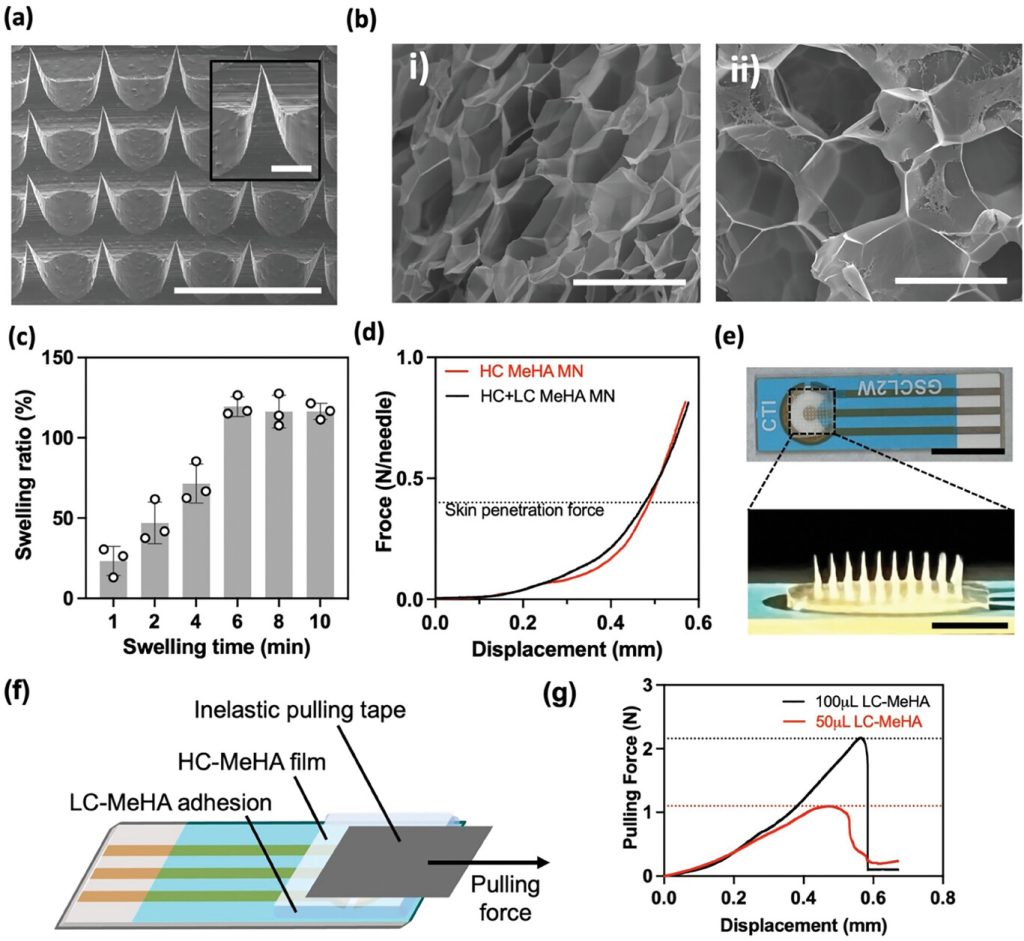
Figure 2. Physical characterization of the HMN patch and integrated Wearable Aptalyzer
The mechanical strength of the HMN patches was evaluated by compression testing to assess their ability to penetrate the skin. The HC+LC-MeHA HMN patches were tested to explore the effect of the addition of LC-MeHA on the mechanical properties of the HMN patches. The displacement of the needle tip after the application of compressive force was measured and plotted. This data shows that each needle of the HC-MeHA HMN and HC+LC-MeHA HMN can withstand forces in excess of 0.3 to 0.4 N, which is necessary to penetrate human skin, without significant needle breakage. Importantly, the inclusion of the LC-MeHA layer did not affect the mechanical strength of the HMN patches.
After characterizing the physical properties of the HMN patch, the HMN patch was integrated with an electrode functionalized with a redox aptamer using LC-MeHA as an adhesion layer. To evaluate the adhesion of the HMN patch to the electrode, a pulling experiment was performed. As shown in Figure 2f, an HC-MeHA film that mimics the HMN patch was attached to the functionalized electrode via a layer of LC-MeHA. A non-elastic tape was then attached, and a lateral force was applied to pull the film away from the electrode, stimulating the force applied to the HMN patch during body movements in real applications.
Wearable Aptalyzer Sensing and Optimization
Prior to validating the performance of the Wearable Aptalyzer for real-world applications, the device fabrication was electrochemically characterized using CV and EIS at open circuit potential. After biofunctionalization with glucose and lactate aptamer probes, the charge transfer resistance of the electrodes increased by 12,366% and 3,073%, respectively. Furthermore, after adding mercaptohexanol (MCH) as a surface blocker for the glucose and lactate sensors, the resistance further increased by 22% and 70%, respectively. Finally, after attaching HMN, the charge transfer resistance increased by 23% and 25%, as expected. CV measurements were also recorded after each fabrication step of the Wearable Aptalyzer. A decrease in electrochemical current was observed after each fabrication step compared to the previous one. This is due to the fact that each layer added during the fabrication process imposes steric hindrance, thereby reducing the access of the electrolyte to the electrode surface. The observed electrochemical current trends are consistent with the charge transfer resistance results calculated by EIS, confirming that each functional layer was successfully added to the device and further validating the successful fabrication of the Wearable Aptalyzer.
Next, the glucose and lactate Aptalyzer sensors were evaluated in vitro using artificial ISF (aISF) spiked with different concentrations of the target analytes. Validation experiments were performed iteratively, starting with a blank aISF sample and then increasing the concentration (upward calibration) or starting with the highest concentration of the target analyte in the aISF and then diluting it (downward calibration). The glucose Aptalyzer was exposed to a concentration range of 0 to 50 mM, covering the normal physiological range, as well as exceeding hypoglycemia and hyperglycemia levels. The lactate range of 0 to 20 mM was chosen to include the physiological range as well as hyperlactatemia. After a 20-minute incubation, the electrochemical signal was measured. During the upward calibration, the Aptalyzer sensor generated an electrochemical signal that increased monotonically with the target analyte concentration, consistent with the operating mechanism of the aptasensor: an increase in target concentration results in a larger electrochemical current due to more frequent interactions of methylene blue with the electrode. The resulting KDM was then calculated to obtain the calibration curves for the glucose and lactate Aptalyzer sensors. A log-linear fit to the glucose calibration curve yielded a limit of detection of 2.4 mM, covering the hypoglycemic range. In addition, by fitting a log-linear line to the lactate calibration curve, a limit of detection of 1.04 mM was achieved. Different behavior was observed during the downward calibration, resulting in limits of detection of 7.44 mM and 4.43 mM for glucose and lactate, respectively.
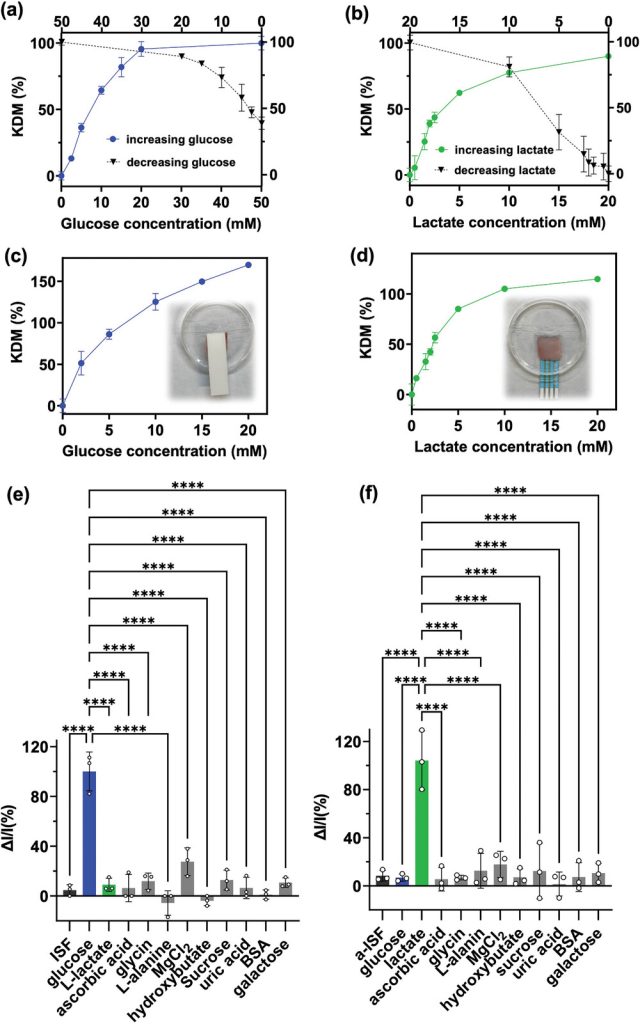 Figure 3. Ex vivo validation of the Wearable Aptalyzer
Figure 3. Ex vivo validation of the Wearable Aptalyzer
To further investigate the integrity of the developed platform, the glucose Aptalyzer sensor was challenged by introducing a panel of common interferents found in ISF, including lactate, ascorbic acid, glycine, L-alanine, magnesium chloride, hydroxybutyrate, sucrose, uric acid, bovine serum albumin (BSA), and galactose, all of which were spiked into aISF. The concentration of all compounds in aISF was 10 mM, except for magnesium chloride, which was 5 mM (the concentration of glucose was within the physiological range, but the concentrations of other interferents were significantly higher than normal). Of all the interferents tested, including sugars and amino acids, none showed interference with the detection of glucose, demonstrating the excellent specificity of the glucose Aptalyzer sensor. Similarly, the selectivity of the lactate Aptalyzer was examined by introducing the same concentrations of interfering compounds found in ISF. The calculated signal gain clearly demonstrated that the lactate Aptalyzer sensor exhibited excellent selectivity for lactate, despite the very high concentrations of non-target molecules.
In vivo validation of the Wearable Aptalyzer using a mouse model
After validating the performance of the Wearable Aptalyzer ex vivo, the researchers evaluated the integrated devices using animal models. Prior to the in vivo evaluation, a quality control procedure based on single-frequency electrochemical impedance measurements was established to identify faulty sensors. Sensors with a |ΔZ/Z| > 500 after insertion into the skin were considered defective and excluded. ΔZ/Z represents the change in electrochemical impedance observed after connecting the HMN to the Wearable Aptalyzer compared to the functionalized sensor. In addition, current cutoffs of -30 nA at 80 Hz for the glucose device and -100 nA at 200 Hz for the lactate device were determined for in vivo testing. A root mean square (RMS) noise value of 0.01 and a signal-to-noise ratio of 3 under signal-on conditions were also determined as critical values for in vivo experiments. Sensor failures may be caused by incomplete connection of the HMN patch to the electrodes or imperfect insertion of the HMN patch into the skin. Continuous glucose and L-lactate measurements were performed on healthy wild-type mice by applying the integrated Wearable Aptalyzer to the dorsal skin. The penetration of the HMN patch was first evaluated by applying the Wearable Aptalyzer for 5 minutes, followed by the collection of skin samples after device removal. Concurrently, blood samples from the mice’s tails were used to measure blood glucose levels with a handheld glucomete. The trends in blood glucose levels measured by the glucometer corresponded well with the glucose KDM values measured by the Wearable Aptalyzer, confirming the device’s reliability in detecting fluctuating glucose concentrations.
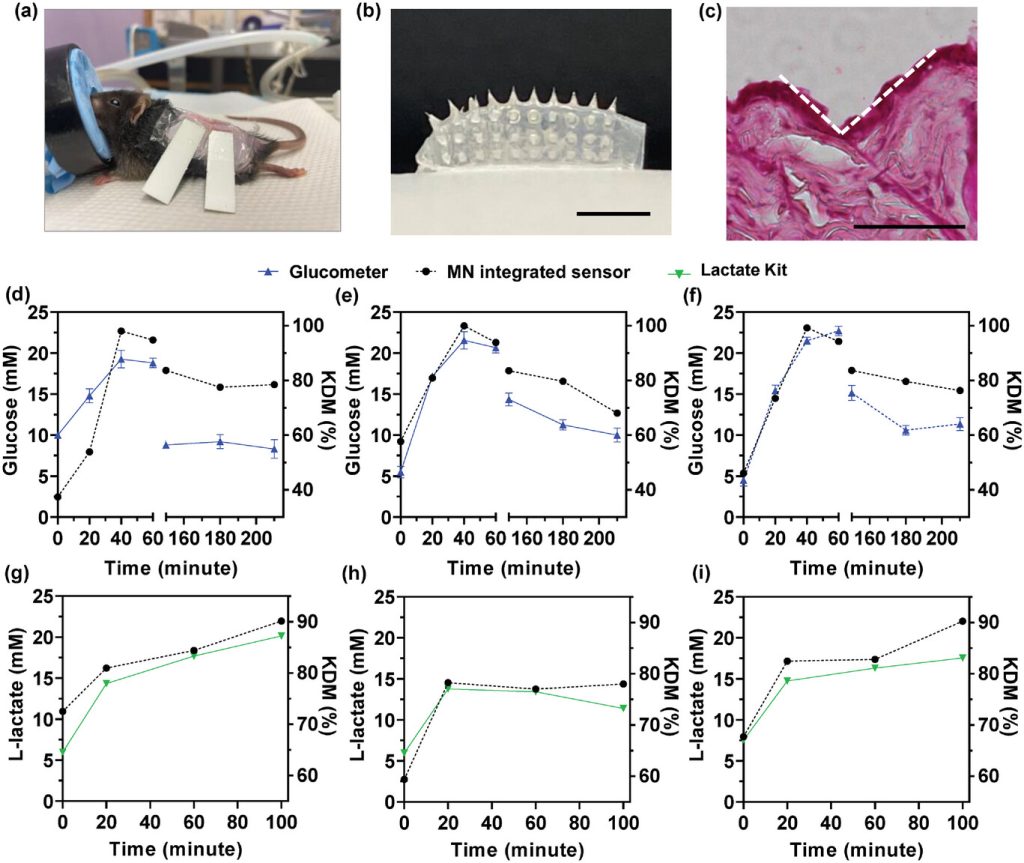 Figure 4. In vivo glucose and lactate detection in mouse models
Figure 4. In vivo glucose and lactate detection in mouse models
In vivo validation of the Wearable Aptalyzer for multiplexed measurements
To evaluate the assay’s potential applicability in various animal models, the Wearable Aptalyzer was further characterized and tested using a diabetic rat model. The device’s effectiveness in penetrating rat skin and the skin’s recovery after HMN application were investigated. Figure 5a shows the Wearable Aptalyzer before and after skin penetration, highlighting the presence of swelling and intact needles. Additionally, the connection between the HMN and the electrode remained secure. The researchers monitored the needle marks on the rat skin after HMN penetration (Figure 5b). Immediately after HMN patch removal, clear needle marks were observed, providing evidence of effective skin penetration. However, the marks disappeared after 20 minutes, indicating rapid skin recovery. H&E staining of the skin revealed a needle cavity with a depth of 92.5 μm within the rat skin (Figure 5c), confirming effective penetration and swift recovery.
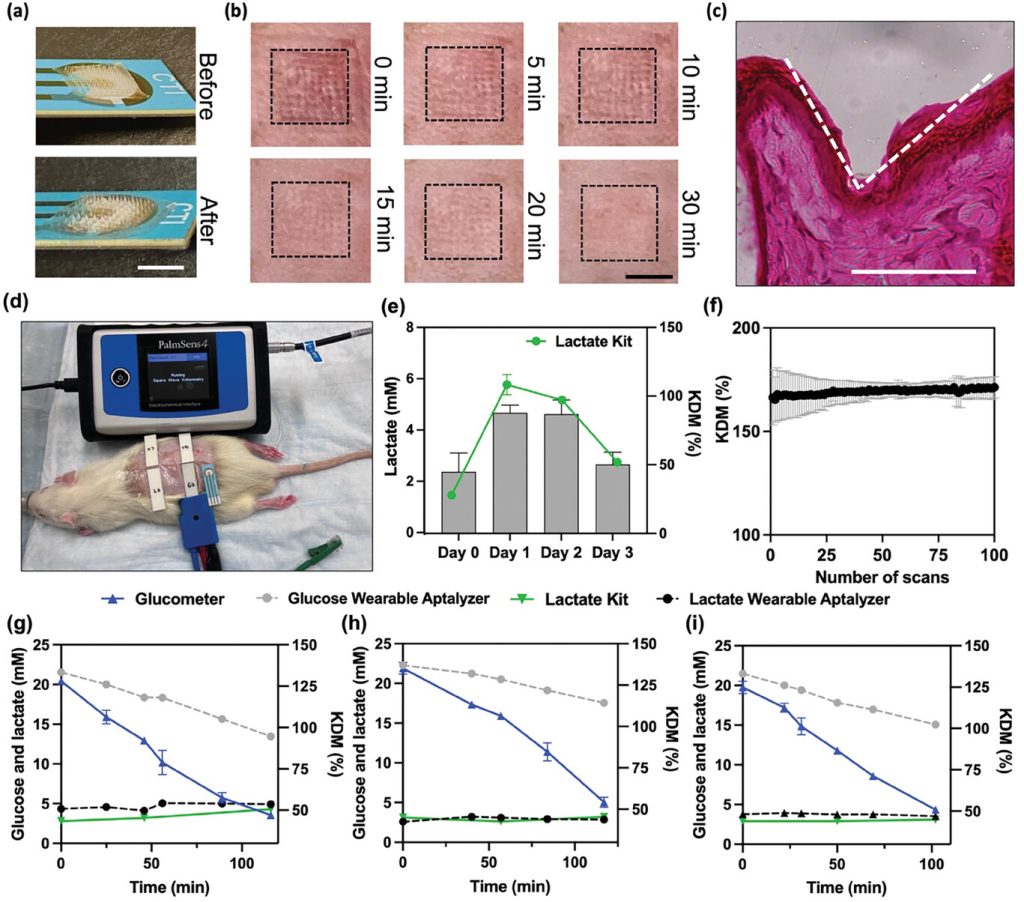 Figure 5. In vivo characterization and multiplexed monitoring using the Wearable Aptalyzer
Figure 5. In vivo characterization and multiplexed monitoring using the Wearable Aptalyzer
Simultaneous, multiplexed measurements of glucose and lactate were performed on diabetic rats using the Wearable Aptalyzer device. Continuous monitoring of both biomarkers allows for accurate assessment of metabolic byproducts related to exercise intensity in diabetic individuals, aiding in predicting glycemic responses and necessary insulin doses. Diabetic rats were fasted for 4 hours before the experiment and then given human recombinant insulin to lower their blood glucose levels. Two lactate and two glucose Wearable Aptalyzer sensors were applied to the shaved backs of the rats, and’ glucose and lactate levels were continuously monitored. Data was collected at five different time points, and the results were cross-validated using a handheld glucometer for glucose and a colorimetric L-lactate kit for lactate. The glucose Wearable Aptalyzer showed a decrease in KDM after insulin injection, while lactate KDM remained consistent, confirming trends similar to blood-based measurements.
Summary
In summary, the researchers report an aptamer-based electrochemical sensor integrated with HMNs for real-time, in vivo analysis of biomarkers in ISF. The Wearable Aptalyzer combines in situ biomarker measurements using ultrasensitive electrochemical readouts with the robust mechanical strength of HMNs for effective skin penetration and ISF sampling. The Wearable Aptalyzer exhibited high sensitivity and specificity in detecting clinically relevant glucose and lactate concentrations in a real skin environment. Its performance was investigated using both mouse and rat models. Importantly, the Wearable Aptalyzer displayed a stable electrochemical signals, enabling continuous lactate monitoring, which correlated with blood lactate levels for at least 3 days in awake diabetic rats. In vivo results from healthy mice and diabetic rats demonstrated the Wearable Aptalyzer’s efficacy in monitoring glucose and lactate trends across different animal models. The simultaneous monitoring of glucose and lactate using the Wearable Aptalyzer highlights its potential for personalized diabetes management. As a universal sensing platform, the Wearable Aptalyzer can track a variety of clinically important biomarkers using different aptamers, which are not achievable enzymatically, demonstrating its potential for a wide range of continuous monitoring applications.
Reference:
Bakhshandeh, Fatemeh et al. “Wearable Aptalyzer Integrates Microneedle and Electrochemical Sensing for In Vivo Monitoring of Glucose and Lactate in Live Animals.” Advanced materials (Deerfield Beach, Fla.), e2313743. 16 May. 2024, doi:10.1002/adma.202313743
Related Services:
One-Stop Microfluidic Solutions