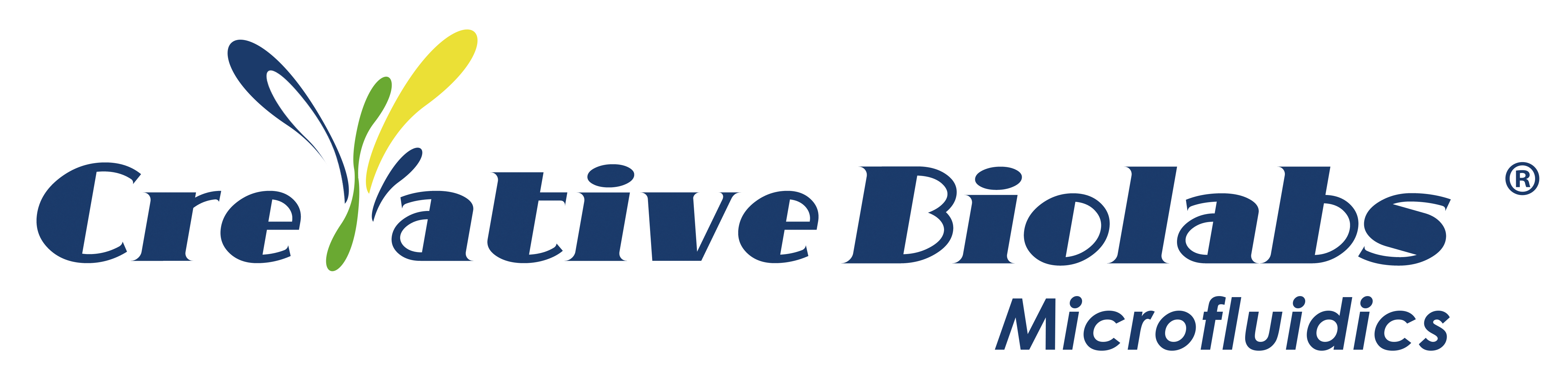In recent years, global public health crises have become increasingly prevalent, necessitating the development of efficient platforms for rapid detection and control their spread. However, traditional diagnostic approaches often involve intricate and time-consuming procedures. Addressing this challenge, the emergence of point-of-care testing (POCT) utilizing portable devices has played a pivotal role in rapidly delivering results, aiding in pandemic response. Microfluidic chips, a crucial innovation in POCT, precisely manage fluid dynamics, mixing, separation, and reactions at the microscale through microchannels and microstructures, thereby improving the accuracy and efficiency of testing. Among them, gravity-driven microfluidic systems, with their versatility and vertical flow capabilities, hold significant promise in this field. Yet, there remains a notable absence of gravity-driven microfluidic platforms capable of autonomously controlling multiple fluids without reliance on external forces.
On December 12, 2023, the online publication of a research article titled “Programmable Gravity Self-Driven Microfluidic Chip for Point-of-Care Multiplied Immunoassays” in the journal “Small” marked a significant advancement.

This research introduces a unique Programmable Gravity Self-Driven Microfluidic Chip (PGSMC), designed to execute sequential releases of multiple reagents, conduct multi-target analyses and facilitate multi-chip operations simultaneously. The initiation of the sequential release process is as simple as vertically flipping the PGSMC, requiring no additional steps or equipment (Figure 1).
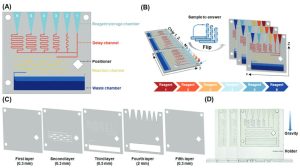 Figure 1. PGSMC Design Schematic
Figure 1. PGSMC Design Schematic
To validate the feasibility of PGSMC’s sequential release, the research team conducted theoretical modeling, numerical simulations, and practical verification. Results indicated that the delay channel, under gravity’s influence, can systematically adjust channel resistance, controlling the sequential release of fluids (Figure 2).
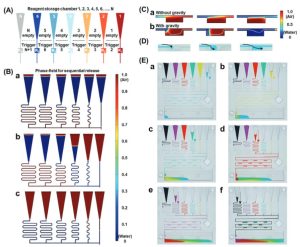 Figure 2. Explanation and Verification of Sequential Release Principle
Figure 2. Explanation and Verification of Sequential Release Principle
To further validate its functionality, PGSMC was applied to multiplex antinuclear antibody testing. Results showed that PGSMC could complete point-of-care immunoassays within 60 minutes, a notable improvement compared to the traditional method (>120 minutes), while maintaining good specificity (Figure 3).
 Figure 3. PGSMC-Based Antinuclear Antibody Immunoassay
Figure 3. PGSMC-Based Antinuclear Antibody Immunoassay
Subsequently, the research team assessed PGSMC’s practical application by analyzing clinical samples, revealing a remarkable 96% sensitivity, 100% specificity and 99% accuracy.
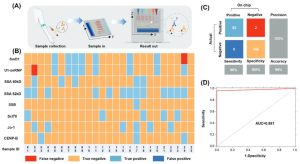 Figure 4. Analytical Performance of PGSMC in Clinical Applications
Figure 4. Analytical Performance of PGSMC in Clinical Applications
In summary, this study introduces a programmable gravity self-driven microfluidic chip characterized by its simplicity and independence from external drivers or equipment. PGSMC achieves programmable multi-reagent release, multi-chip operation, and multi-target analysis for high-throughput testing. Users can effortlessly obtain results by simply adding reagents. Notably, the antinuclear antibody immunoassay was completed in less than 60 minutes, showcasing superior analytical performance compared to the traditional method (>120 minutes) and demonstrating good analytical performance. The evaluation on 25 clinical samples demonstrated excellent sensitivity (96%), specificity (100%) and accuracy (99%). This research offers innovative ideas for POCT, particularly in the realm of automated fluid control.
Reference:
Yuan H, Wan C, Wang X, et al. Programmable Gravity Self-Driven Microfluidic Chip for Point-of-Care Multiplied Immunoassays. Small. Published online December 12, 2023. doi:10.1002/smll.202310206
Related Services:
One-Stop Microfluidic Solutions