On May 31, 2023, researchers from the Hong Kong University of Science and Technology published a review article titled “Vascularized Human Brain Organoid Chips” in the esteemed journal Lab on a Chip. This comprehensive review delves into the intricate world of in vivo human brain development and introduces a state-of-the-art in vitro human brain model. The paper elucidates s various strategies aimed at enhancing the microenvironment of vascularized human brain organoids using microfluidic devices, Additionally, it addresses the existing challenges and envisions future directions in the organoid and microfluidics domain, paving the way for innovative solutions to overcome emerging obstacles in this field.
In Vivo Biology of Human Brain and Animal Models
To create physiologically accurate in vitro vascularized brain organoids-on-a-chip, a deep understanding of critical stages in human brain development is indispensable. Neurogenesis and vascularization mainly occur during the embryonic period. Neurogenesis involves the transformation of neuroepithelial cells into cortical neurons, which subsequently organize into six distinct cortical layers through differentiation and migration. Simultaneously, vascularization takes place as the mesoderm-derived vascular network infiltrates the developing brain. This process is followed by continuous sprouting and remodeling to establish the blood-brain barrier (BBB). Dysfunctions in the BBB have been linked to various neurological disorders. While animal models provide valuable insights, differences from the human brain limit the clinical translation of results.
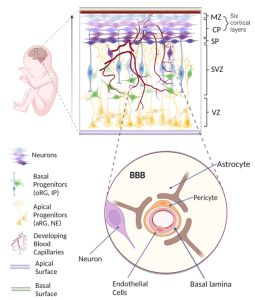 Figure 1. Human Brain Development In Vivo
Figure 1. Human Brain Development In Vivo
Advancement In Vitro Human Brain Model
The ongoing evolution of in vitro human brain models has indeed expanded their potential to complement animal models; however, they do not fully replicate the intricate complexity of the human brain accurately. To address this limitation, validation testing in combination with animal models and human studies is imperative. Efficient modeling of the human brain, particularly with a tightly controlled BBB, remains a significant bottleneck in drug discovery and the comprehension of human brain diseases. The Transwell system, comprising two compartments separated by a porous membrane, serves as a simplified model of the human BBB but lacks dynamic features. In contrast, microfluidics offers a superior advantage over Transwell systems by simulating the realistic environment of human blood flow (Figure 2A). This system facilitates the integration of transepithelial electrical resistance (TEER) electrodes, enabling real-time monitoring and quantification of barrier function. However, the up-down configuration might hinder simultaneous real-time imaging of all BBB cell types.
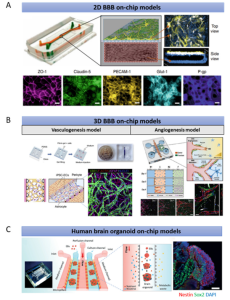 Figure 2. Existing Microfluidics-Based Models of the Human Brain
Figure 2. Existing Microfluidics-Based Models of the Human Brain
While 2D BBB chips capture essential elements of cell biology, they lack a functional 3D brain tissue environment and are unsuitable for studying tissue-level biological processes and systems. The 3D BBB chip, utilizing polydimethylsiloxane (PDMS)-based photolithography, creates spaced micropillars forming open lumens of blood vessels, thus closely simulating the in vivo BBB (Figure 2B). Neurovascular unit (NVU) chips integrate multiple cell types and appropriate flow simulations for drug research and metabolite analysis. Nevertheless, these models encounter challenges problems such as limited cell variety, short life cycles, and the inability to reliably simulate specific BBB-related diseases. Generating brain organoids containing diverse cell types may offer a better approximation of human brain physiology. One study investigated the effects of prenatal nicotine exposure on cerebral organoids using perfusion flow (Fig. 2C). However, the impact of perfusion flow on neuronal activities, such as synchronized bursts and spikes, was only observable in two-month-old organoids. Long-term cultivation of brain organoids is necessary to mirror brain aging phenotypes and enhance our understanding of the aging process. The question of whether perfusion flow can mitigate advanced cerebral organoid necrosis remains unanswered. Most significantly, none of these models incorporate vasculature, a crucial component of the intricate human brain system.
Strategies for Creating Vascularized Brain Organ-on-Chips with Physiological Microenvironments
Numerous existing brain chips can recreate the human BBB environment at the cellular level, while brain organoids can mimic the human brain at the organ level. Although structurally distinct, brain organoids and BBB-on-a-chip technologies encounter similar technical and biological challenges. Combining these approaches holds the potential to develop vascularized brain organoid-on-a-chip models. Importantly, vascularization of brain organs could be achieved by establishing a BBB microenvironment on a chip. Successful vascularization of kidney and liver organs has been demonstrated using chip technology. Similarly, vascularization of the brain organ-on-a-chip models can be enhanced further by integrating them with a perfusable vascular network on-chip, enabling efficient material delivery (Figure 3).
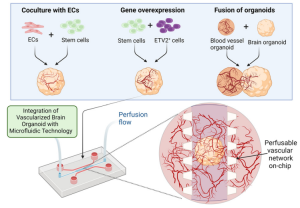 Figure 3. Enhanced Vascularization of Brain Organoids Using Microfluidics
Figure 3. Enhanced Vascularization of Brain Organoids Using Microfluidics
Currently, vascularization of brain organoids has been achieved through three main methods: co-culturing endothelial cells (ECs) with stem cells, overexpressing specific genes, and fusing brain and vascular organoids. These methods can be combined with microfluidic technologies to generate perfusable cerebral vascular networks. Incorporating perfusion flow into the system simulates physiological conditions. Moreover, improving vascularized brain organoids on a chip involves introducing biophysical cues such as shear stress induced by transmural flow and interstitial flow on the chip. The duration, frequency, and amplitude of mechanical forces are crucial factors in simulating physiological mechanical conditions. Although the effects of mechanical stimulation on vascularized brain organoids have yet to be fully elucidated, existing studies on how these factors affect brain organoids underscore their importance in constructing fully functional vascularized brain organoids-on-a-chip (Figure 4).
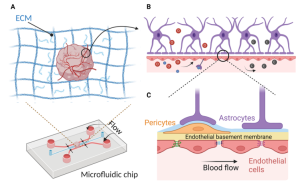 Figure 4. Vascularized Brain Organoid-on-a-Chip Microenvironment for Simulating Physiological Conditions
Figure 4. Vascularized Brain Organoid-on-a-Chip Microenvironment for Simulating Physiological Conditions
Challenges and Future Directions of Vascularized Brain Organoid Chips
Despite the immense potential of advanced in vitro models for understanding various mechanisms related to blood vessel-brain interactions, a substantial gap still exists between current organoid-on-a-chip technology and in vivo brain development. Future research should focus on enhancing existing models and integrating different strategies (Figure 5). A key challenge in vascularized brain organoids-on-a-chip is the precise regulation of the balanced microenvironment of brain organoids and perfusable vascular networks. Tailored biochemical cues must be designed and optimized to integrate these diverse cultures on a chip. While sensors can monitor rapid physiological and biochemical changes within microfluidic environments, their integration into brain organoids remains technically challenging due to their large size. Future developments should explore effective methods to seamlessly integrate sensors into microfluidic systems. Moreover, achieving high-throughput vascularized brain tissue culture on a chip necessitates considering technical stability and the reproducibility of organoid cultures. Future directions should streamline the mechanical automation process on the chip and minimize reliance on manual operations.
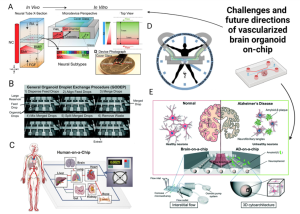 Figure 5. Challenges and future directions for vascularized brain organoids-on-a-chip.
Figure 5. Challenges and future directions for vascularized brain organoids-on-a-chip.
Most brain organoid studies to date have overlooked a crucial aspect of the human brain: circadian rhythms, which regulate brain behavior and physiology. Establishing on-chip rhythms by introducing chemical messengers with hormone-regulating effects and integrating them with vascularized brain organoid-chip models to achieve sustained circadian behavior will enhance the system’s predictive value for clinical applications. In addition to disease modeling, vascularized brain organoid models hold significant potential for regenerative medicine. However, challenges such as low transplantation efficiency, high costs, and ethical and safety concerns persist. Current solutions aim to reduce brain organoid cultivation costs by using patient-derived stem cells for personalized medicine and employing cost-effective synthetic hydrogels. Lastly, while individual organoid chips serve as powerful platforms for modeling specific organs, the development of multiple organoid chips interconnected through a perfusable vascular network is vital for precise assessments of multi-organ interactions. Therefore, multi-organoid microfluidic chips containing vascularized brain organoids and other vascularized organoids simultaneously connected through a perfusable vascular network, hold greater significance for diverse clinical applications.
Reference:
https://doi.org/10.1039/D2LC01109C
Related Services: