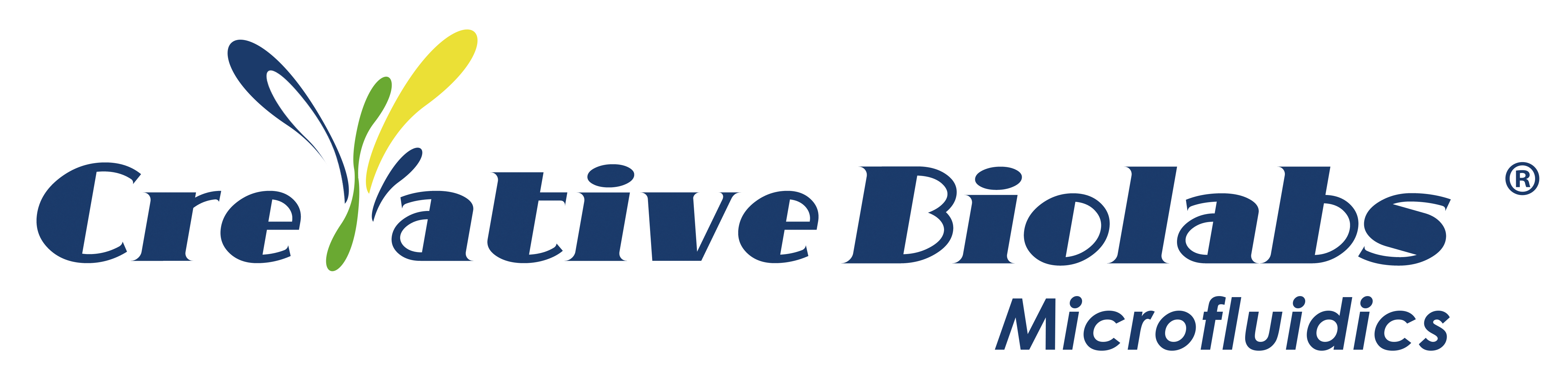Protein analysis at the single-molecule level reveals heterogeneous behaviors that are often obscured by ensemble averaging techniques. Digital quantification of enzymes typically involves the observation and counting of individual molecules segregated into microdomains through the conversion of fluorescent substrates. However, this strategy, reliant on linear signal amplification, is limited to a handful of enzymes exhibiting sufficiently high turnover rates.
Recently, researchers from ESPCI Paris-PSL Research University in France proposed an alternative technology rooted in molecular programming and droplet microfluidics the digital detection of various DNA and RNA processing enzymes. The relevant research results were published in the journal Nature Nanotechnology under the title “Functional analysis of single enzymes combining programmable molecular circuits with droplet-based microfluidics”.

The study utilized a versatile molecular circuit known as a programmable ultrasensitive molecular amplifier (PUMA), which exponentially amplifies DNA signals based on a threshold and links them to various input activities. PUMA includes a conversion module that connects the target (in this case, enzyme activity) to the production of a short DNA signal strand, and a DNA-enzyme amplification system that ultimately produces an intense fluorescence signal. The DNA-enzyme amplification system consists of three DNA templates: DNA polymerase (Vent (exo-)) and nicking enzyme (Nb.BsmI) work together to create an autocatalytic template for exponential replication of the catalytic signal chain; a false template deactivates a portion of the signal strand, acting as a catalytic drain to avoid non-specific, non-target-dependent amplification resulting from leakage reactions; while the pre-fluorescent reporter template (rT) hybridizes with the signal strand and generates a fluorescent signal upon polymerization. In addition, the researchers designed a conversion module that connects the amplification switch to the activity of the nickase Nt.BstNBI (NBI for short) activity for diagnostic purposes. The researchers monitored the response in real time as NBI concentrations increased. As expected, higher target concentrations led to quicker generation of the signal chain and consequently initiated exponential amplification more rapidly. The overall sensitivity of this method is approximately 1 μ/ml (milliunits per milliliter, or approximately 400 fM of active enzyme), which is four orders of magnitude lower than assays relying on linear conversion of fluorescent substrates.
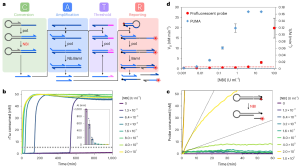
Figure 1. Detection Principle of NBI Nickase Activity
Taking advantage of the high-sensitivity properties of PUMA-based assays, the researchers envisioned a comprehensive technique dubbed digital PUMA (dPUMA) for the precise quantification of single enzymes. To evaluate this approach, they introduced varying concentrations of the target enzyme into the NBI circuit, which was subsequently partitioned into monodispersed droplets. These droplets were then subjected to amplification and scrutinized using end-point fluorescence microscopy. By quantifying the proportion of positive droplets and assuming a random, Poisson distribution, the concentration of active enzyme was accurately determined. Notably, this concentration was found to be directly proportional to the initial input concentration across a wide range of dilution series, confirming the method’s ability to digitally detect the enzyme with precision. The dPUMA workflow seamlessly integrates with all one-pot assays, rendering it suitable for the absolute quantification of a wide range of enzymatically active molecules. Strikingly, the numerical outputs revealed a striking discrepancy between the concentration of catalytically active enzyme and the total enzyme concentration, often spanning several orders of magnitude. After ruling out potential experimental artifacts—such as enzyme inactivation or loss during emulsification or the presence of substantial quantities of contaminating proteins—the researchers concluded that the sample indeed harbored significant amounts of non-functional enzymes. These enzymes likely stemmed from various stages of protein production, purification, and possibly inactivation during storage.
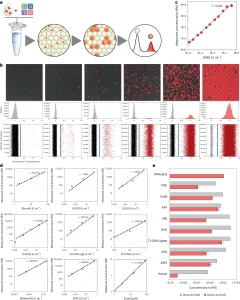 Figure 2. Digital Detection of DNA-Related Enzymes
Figure 2. Digital Detection of DNA-Related Enzymes
To further investigate the composition and evolution of the enzyme mixture, the researchers evaluated the activity distribution of a theoretically identical peptide library (commercial NBI) using a real-time version of the dPUMA protocol. Emulsifying samples with varying enzyme concentrations allowed for the measurement of the activity distribution at different Poisson parameters (λ). Within each occupied droplet, the onset time served as a proxy for enzyme activity: the more active the enzyme, the quicker the amplification—hypothesis confirmed by the monotonic relationship between onset time and enzyme activity observed in batch measurements . As illustrated in Figure 3f, the amplification time at low λ (<2) exhibits a narrow range, facilitating the differentiation of droplet spikes with different occupancies (one, two, three or four enzymes). The researchers verified that the activity distribution was aptly described by the sum of Gaussian functions, with their respective weights constrained by the Poisson frequency of the lambda measurements in each sample. The alignment between droplet occupancy and Poisson’s law demonstrates that the low activity fraction measured by digital analysis is not due to a non-Poisson distribution of the enzyme—a phenomenon that can occur with enzymes prone to aggregation or binding to long stretches of genomic DNA. Crucially, the coefficient of variation of the onset time distribution in droplets containing a single enzyme was merely 17%, indicating a relatively uniform distribution of activity within the active enzyme pool.
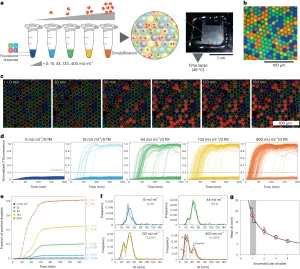 Figure 3. Evaluation of Peptide Library Activity Distribution Through Time-Lapse Experiments
Figure 3. Evaluation of Peptide Library Activity Distribution Through Time-Lapse Experiments
To explore the origins of the inactive components, the researchers embarked on evaluating how the activity distribution was affected under various stress conditions—namely,physical stress such as a heat shock step, or chemical stress. In the case of physical stress, characterized by, an exponential decay of the active fraction of the enzyme with increasing heat shock duration, a noteworthy finding emerged: despite the decline in the active fraction, the activity distribution within the residual active fraction remained strikingly akin to that of the untreated sample. This phenomenon of two-state behavior is observed regardless of the heat shock temperature, wherein the enzyme exhibited either full activity or complete inactivity. Such behavior aligns the concept of the catastrophic denaturation model described in previous reports, where a heated enzyme undergoes reversible conformational changes until it reaches a critical point, leading to irreversible unfolding. Conversely, during oxidative treatment, a discernible pattern unfolded: with increasing H₂O₂ concentration, the proportion of active enzymes once again dwindled. However, a notable deviation surfaced in the distribution of onset times compared to the untreated sample: heightened oxidative stress correlated with an increased number of single enzymes exhibiting lower but still measurable activity. This observation suggests that mild oxidative stress results in a fraction of inactive enzymes alongside a cohort of enzymes exhibiting moderate activity. This hints at a mechanism whereby oxidative substrates with reduced activity become accessible before complete loss of activity. Oxidation of side chains of residues outside the active site could potentially destabilize the catalytic pocket, impede substrate recognition, or diminish scaffold stability. Importantly, this broadened activity distribution was conspicuously absent in untreated commercial samples, hinting at the presence of an inactive fraction of the full-length polypeptide possibly linked to folding/aggregation issues rather than chemical damage.
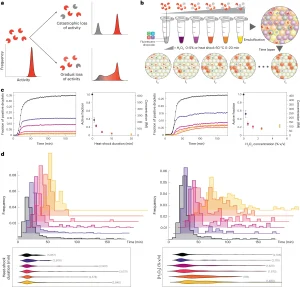 Figure 4. Study on the Functional Heterogeneity of Enzyme Groups
Figure 4. Study on the Functional Heterogeneity of Enzyme Groups
Taken together, by combining the sensitivity of exponential molecular amplifiers with the modularity of DNase circuits and droplet readouts, researchers can specifically detect nearly all D(R)NA-related enzymatic activities at the single-molecule level. . This strategy, called digital PUMA (Programmable Ultrasensitive Molecular Amplifier), has proven effective across a spectrum of enzymes, including even those with slower catalytic rates, and has pushed boundaries to the apparent singleton level of Streptococcus pyogenes Cas9, stretching the turnaround limit. The unique digital counting method facilitated absolute molar quantitation and unmasked substantial quantities of inactive catalysts across all tested commercial formulations. Furthermore, by meticulously tracking the amplified reactions of individual enzyme molecules in real time, the researchers also extracted the activity distribution within the catalyst population, revealing additional deactivation pathways under various pressures. This approach significantly broadens the scope of enzymes amenable to single-molecule resolution quantification and functional analysis, thus positioning digital PUMA as a versatile framework for precise enzyme quantification in diagnostic or biotechnology applications. Additionally, this digital assay holds promise for delving into the origins of protein functional heterogeneity.
Reference:
Gines G, Espada R, Dramé-Maigné A, Baccouche A, Larrouy N, Rondelez Y. Functional analysis of single enzymes combining programmable molecular circuits with droplet-based microfluidics. Nat Nanotechnol. 2024 Feb 26. doi: 10.1038/s41565-024-01617-1. Epub ahead of print. PMID: 38409552.
Related Services:
Microfluidic Development Services for Droplet Generator and Flow Chemistry
Microfluidic Development Service for Enzymes/Inhibitors Assay