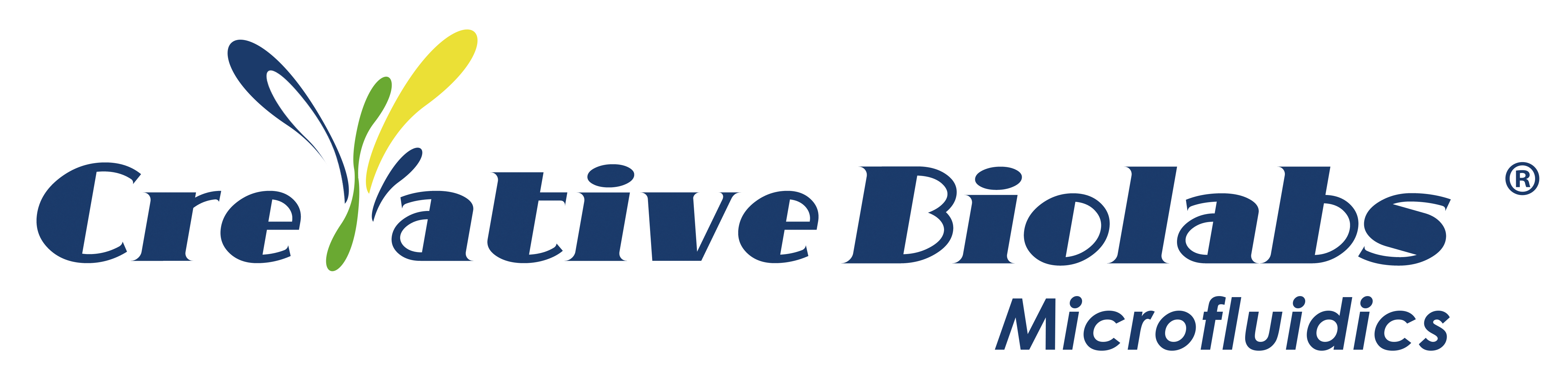Nanomedicine involves the medical application of nanotechnology, utilizing nanoscale or nanostructured materials in medical applications such as biosensing/bioimaging, disease diagnosis, and drug delivery, etc. Compared to conventional drugs, nanomedicines offer potential enhancements in bioavailability, targeting ability, delivery efficacy, dose response and personalized treatment, and have broad application prospects. Evaluating the in vivo metabolism, transport, and toxicity of nanodrugs is essential before their clinical use and market release. Differences in physiological responses between animal models and humans may lead to significant biases in understanding the efficacy and toxicity of nanodrugs. Additionally, commonly used two-dimensional (2D) monolayer cell culture model also has differences in mechanical stress and microenvironment compared with normal in vivo cells and tissues, making them inadequate for accurately predicting the in vivo toxicity and other biological effects of nanomedicines.
Microfluidic Organ-on-a-Chip for Nanomedicine Evaluation
In nanomedicine evaluation, dynamic 3D cell cultures with fluid flow clearly offer additional advantages over static 2D models. First, 3D cultures can simulate cell-cell or cell-extracellular matrix interactions, providing a better bionic platform. Secondly, the combination of microfluidic technology allows continuous nutrient exchange and adequate oxygen perfusion, better simulating in vivo conditions. Thirdly, multiple steps such as cell culture, sampling, capture, lysis, imaging and detection can be integrated into a single system, making nanomedicine evaluation more efficient and convenient.
 Timeline of the development of microfluidics-based Organ Chip technology.
Timeline of the development of microfluidics-based Organ Chip technology.
An organ chip is a microfluidic cell culture device created through microchip fabrication methods. It contains continuously perfused chambers for cell culture, designed to mimic physiological conditions at the tissue level or organ level. Organ chips enable precise evaluation of the transport and transfer of nanomedicines between tissues and tissue interfaces under relevant shear flow and can also reveal the biological mechanisms and adverse effects of nanodrug targeting by monitoring subtle changes in different parts of organs/tissues. In recent years, organ chip development has rapidly covered several major organs and disease areas, reducing the cost of nanomedicine development and avoiding adverse impacts on animals and humans during trials. The following is an inventory of the latest developments in various organ chip models, with a focus on their applications in nanodrug evaluation.
Respiratory System
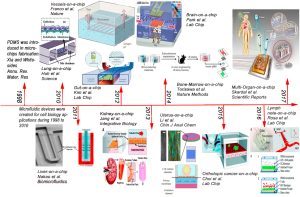
The respiratory system serves as the primary gateway for nanoparticles entering the human body, and various natural and engineered nanoparticles are known to potentially cause lung diseases. The pioneering term “lung-on-chip” was introduced by Huh et al. in 2010. The biomimetic lung-on-a-chip microsystem reconstructs the alveolar-capillary barrier, the smallest functional unit in the lung (Figure A and B). To reproduce the air-liquid interface, a dual-channel microfluidic device based on polydimethylsiloxane (PDMS) was used, with a porous membrane coated with collagen separating the alveolar epithelial cells in the upper channel, in contact with air, from the microvascular endothelial cells in the bottom channel, perfused with cell culture medium. In addition, a vacuum channel was included beside the fluid channel to induce cyclic strain at the alveolar-capillary interface, simulating physiological respiratory motion. This biologically inspired microdevice reproduces multiple complex physiological organ-level responses, including lung inflammation reactions induced by bacteria or inflammatory cytokines introduced into the alveolar space. Under cyclic strain, the epithelial cells of the lung chip were exposed to silica nanoparticles with a diameter of 12 nm for 5 h (Figure C), revealing an increase in nanoparticle transport from epithelial cells to the endothelial channels (Figure D). The expression of the intercellular adhesion molecule-1 (ICAM-1) and the production of reactive oxygen species (ROS) were significantly increased (Figures E and F below), indicating enhanced pro-inflammatory behavior of nanoparticles with increased respiratory action, exacerbating the development of acute pneumonia. These results imply that traditional static cell culture systems may underestimate the effects of nanoparticles in assessing nanoparticle toxicity.
 Application Example of Lung-on-a-Chip in Nanomedicine: Study of Nanoparticle Migration and Inflammatory Effects
Application Example of Lung-on-a-Chip in Nanomedicine: Study of Nanoparticle Migration and Inflammatory Effects
Recent research has also pioneered lung-on-a-chip models co-culturing primary lung epithelial cells and vascular endothelial cells. In physiologically relevant microenvironments and layered structures, these models can simultaneously assess damage to epithelial and endothelial cells caused by nanoparticles. In addition to toxicity testing, lung-on-a-chip can be developed to evaluate nanodiagnostics and nanotherapeutics. Future research can also focus on integrating more cell types found in the alveoli, such as fibroblasts and alveolar macrophages, and representing oxygen gradients more accurately in the human lung microenvironment.
Digestive System
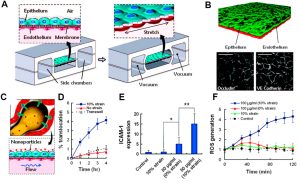
The digestive system serves as a potential pathway for various nanomedicines, either through intentional ingestion or indirect ingestion through inhaled particles. This system comprises hollow organs such as the mouth, esophagus, stomach, small intestine, large intestine, and anus, as well as solid organs like the liver, pancreas, and gallbladder. So far, liver chips combined with intestinal chips have been utilized in specific nanomedicine studies. The initial liver-on-a-chip model primarily focused on drug toxicity screening and consisted solely of liver cells lacking intercellular interactions. Recent advancements have led to the development of a co-culture liver-on-a-chip system that integrates four major types of hepatocytes—sinusoidal endothelial cells, Kupffer cells, hepatic stellate cells, and hepatocytes. These cell types are segregated by permeable membranes, creating adjacent channels that mimic the vital structures and functions of liver sinusoids. Esch et al. successfully merged a liver chip with an intestinal chip to assess nanoparticle toxicity within the digestive system (as illustrated below), highlighting the significance of multi-organ chips in evaluating nanomedicine toxicity. Although existing studies predominantly employ immortalized cell lines, future research should transition towards primary cells to establish a more authentic physiological microenvironment, enhancing our understanding of nanomedicine delivery and its effects.
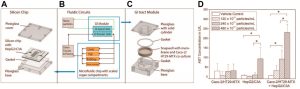 Examples of Liver-on-a-Chip and Gut-on-a-Chip Applications in Nanomedicine: Nanoparticle Ingestion Enhances Liver Damage Through Crosstalk Between Gastrointestinal and Liver Tissues
Examples of Liver-on-a-Chip and Gut-on-a-Chip Applications in Nanomedicine: Nanoparticle Ingestion Enhances Liver Damage Through Crosstalk Between Gastrointestinal and Liver Tissues
Lymphatic System
The lymphatic system comprises organs such as blood vessels, lymph nodes, spleen, tonsils, and thymus. Delivering drugs through the lymphatic system offers the advantage of bypassing first-pass metabolism in the liver and targeting diseases that spread through the lymphatic system. Presently, spleen chips find applications in nanomedicine. The spleen, the smallest functional unit of the red pulp, primarily functions in blood filtration. In severe illnesses or traumatic situations, the spleen may struggle to clear pathogens entirely, leading to fatal sepsis. Therefore, microfluidic artificial spleen devices were developed to clear pathogens from sepsis patients’ blood (as shown below). These devices employ magnetic nanoparticles via mannose-binding lectin (MBL) (Figure A below) capable of encapsulating diverse pathogens, such as Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) (Figure B below). Furthermore, the device replicates the spleen’s intricate structure, including a high-flow vascular arterial channel perfused with contaminated whole blood, a parallel low-flow or intermittent flow sinus channel, and a slit connecting the two, simulating arterial cords and venous systems separated by sinusoidal spaces (Figure C below). As magnetic nanobeads mix with contaminated blood and flow through the microchannels, magnetic separation eliminates pathogens through the venous sinus channels, leaving purified blood in the arterial channels. Experiments have validated the device’s efficiency in removing various bacteria, fungi, and endotoxins from human blood.
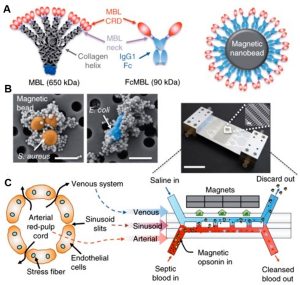 Application Example of Spleen-on-a-Chip in Nanomedicine: Biospleen Device for Blood Cleaning
Application Example of Spleen-on-a-Chip in Nanomedicine: Biospleen Device for Blood Cleaning
Human spleen’s interendothelial intercellular gaps are approximately 200 nm, with nanoparticles larger than 200 nm expected to accumulate in the spleen. Future studies could explore the behavior of deformable nanoparticles and employ dynamic on-chip models for a comprehensive understanding of accumulated nanoparticles’ interaction with the spleen. Additionally, investigating the role of the spleen’s biological cell components in homeostasis and pathological filtering processes represents a related development direction for this organoid chip.
Excretory System
The kidney serves as the primary excretory organ in the human body, comprising multiple cell types, including glomerular vascular endothelial cells and podocytes. The kidney possesses drug-metabolizing abilities, with proximal renal tubule epithelial cells being the most sensitive targets of nanomedicines. Although no studies have reported the use of kidney chips for nanotherapeutic applications, a related study involves nanoparticles as imaging adjuvants for kidney injury (as shown below). Gamma-glutamyl transpeptidase (GGT) is present on the apical membrane of proximal tubule cells and is released after cytotoxic injury. Introducing 500 nm fluorescent polystyrene nanoparticles conjugated with anti-GGT antibodies in the top channel enables nanoparticle aggregation through immunocapturing released GGT. Measuring fluorescence in the effluent facilitates tracking drug-induced nephrotoxicity (Figure A below). Innovative integration with a smartphone enables real-time, interference-free monitoring of the kidney chip both internally and externally (Figure B below).
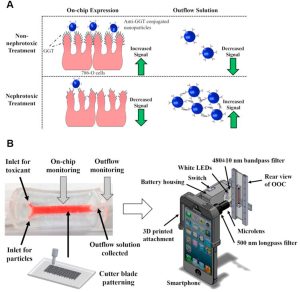 Incorporation of Nanoparticles into a Kidney Chip Enables In Situ Monitoring of Nephrotoxicity
Incorporation of Nanoparticles into a Kidney Chip Enables In Situ Monitoring of Nephrotoxicity
In recent years, significant strides have been made in biomedical research, particularly in the development of innovative strategies such as 3D bioprinting and induced pluripotent stem cell (iPSC)-derived podocytes. These advancements have paved the way for the creation of intricate kidney-on-a-chip platforms. Looking ahead, the future of research lies in integrating various cell types and functions to conduct comprehensive studies, particularly in nanoparticle clearance, specifically focusing on nanoparticles smaller than 10 nm in diameter, whica can be swiftly cleared by the kidneys.
Nervous System
The blood-brain barrier (BBB) stands as a crucial line of defense, safeguarding the brain from foreign substances while also posing challenges in the delivery of beneficial compounds. Overcoming this barrier has become a pivotal goal in nanomedicine. Recently, a microfluidic BBB model was devised to evaluate Ang2-conjugated liposome nanocarriers (Ang2-Liposomes) by brain endothelial cells, as well as subsequent BBB penetration (as shown below). Ang2, a peptide ligand for low-density lipoprotein receptor-related protein 1 (LRP1) on brain endothelial cells, facilitates BBB penetration. Intriguingly, the study revealed that applying shear stress significantly enhanced Ang2-liposomes’ penetration into the BBB, surpassing the efficacy of static incubation.
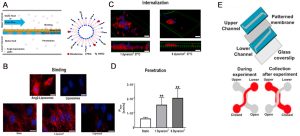 Examples of BBB Chip Applications in Nanomedicine: Assessing Ang2-Liposomes Binding, Internalization, and Penetration
Examples of BBB Chip Applications in Nanomedicine: Assessing Ang2-Liposomes Binding, Internalization, and Penetration
Further advancements were made by enhancing the barrier function of human iPSC-derived brain microvascular endothelial cells using hypoxic conditions. These biomimetic models, with heightened transendothelial resistance (TEER) values, hold promise for guiding the design of nanoparticle therapeutics. These models can ensure efficient binding and internalization by brain endothelial cells amidst dynamic blood flow while maximizing BBB penetration. Additionally, these models offer avenues for studying particle behavior, interaction patterns, and toxic effects of nanomedicines.
Vascular System
Intravenous injection remains the gold standard for systemic drug delivery, ensuring an impressive 100% bioavailability. By bypassing efflux pumps and first-pass metabolism, it ensures the efficient distribution of drugs and nanoparticles into the systemic circulation. Studying the intricate interplay of nanomedicines with the vasculature, alongside understanding vascular transport and toxicity, has become paramount. The vascular chip model can deliver fluid shear stress and cyclic stretch to vascular cells simultaneously or independently. This unique capability simulates the hemodynamic microenvironment of blood vessels within the human body. Beyond merely exploring nanoparticle interactions with endothelial cells, microfluidic models offer an additional advantage. They validate the potential of shear-responsive nanoparticles in targeting and treating obstructed blood vessels (as shown below). In this example, microfine aggregates of nanoparticles were engineered into a thrombolytic delivery system. Specifically designed to deliver to regions of obstructed blood flow, these aggregates respond to abnormally high fluid shear stress. Under these conditions, they break down into nanoscale components, concentrating and releasing the drug specifically at the site of thrombosis. The effectiveness of this delivery system was rigorously tested in an ex vivo mouse pulmonary embolism model, showcasing the potential of stimulating nanoengineering through pathophysiological mechanisms. This approach paves the way for the development of safer and more effective treatment strategies.
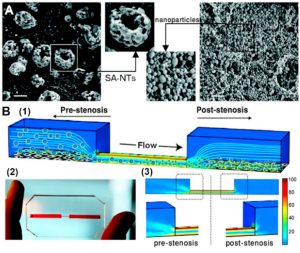 Example of Vascular Chip Applications in Nanomedicine: Validating Shear-Responsive Nanoparticles to Target and Treat Obstructed Blood Vessels
Example of Vascular Chip Applications in Nanomedicine: Validating Shear-Responsive Nanoparticles to Target and Treat Obstructed Blood Vessels
Furthermore, blood vessels play a pivotal role in connecting various organs in the human body, facilitating the transport of essential nutrients, oxygen, hormones and nanomedicines. Recognizing their significance, future research endeavors should integrate the vascular system into other organ-on-a-chip microfluidic models. This integration aims to simulate more realistic physiological responses. Moreover, recent studies have shed light on the impact of particle shape and size on target specificity and non-specific accumulation.
Summary and Discussion
As promising candidates for in vitro screening of nanomedicines, organ-on-chips offer unparalleled control over biomechanical, biochemical, and biophysiological microenvironmental cues. These cues are instrumental in replicating key physiological conditions and characteristics, holding immense potential for advancing medical research. However, amidst the optimism, significant challenges persist. One notable limitation lies in the scope of 3D organ chips, which, while informative, only capture specific aspects of real tissues. Additionally, single-chip platforms are confined to the functions of specific organs, replicating the complex structures of human tissues and organs in a miniaturized format remains a formidable challenge.
Reference
Chen X, Zhang YS, Zhang X, Liu C. Organ-on-a-chip platforms for accelerating the evaluation of nanomedicine. Bioact Mater. 2020;6(4):1012-1027. Published 2020 Oct 12. doi:10.1016/j.bioactmat.2020.09.022
Related Services: