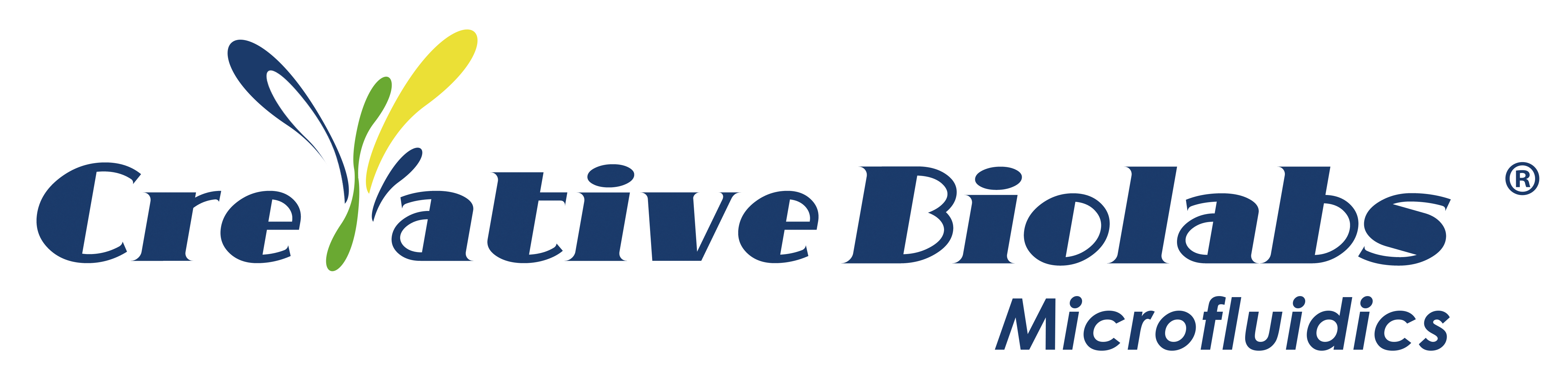On June 28, 2023, a research paper titled “Development of a Human Malaria-on-a-Chip Disease Model for Drug Efficacy and Off-Target Toxicity Evaluation” was published in the prestigious journal Scientific Reports. This study introduced a functional, serum-free multi-organ system for cultivating Plasmodium falciparum, aiming to establish an innovative platform for drug development. The multi-organ chip presented a novel approach to evaluating malaria therapeutics within the human system. It enabled simultaneous enabling the assessment of efficacy and safety, providing the field of malaria research with an innovative and efficient tool for preclinical studies and the discovery of antimalarial drugs.

Multi-Organ Microfluidic System Design
In this study, a serum-free microfluidic device was developed, comprising three independent chambers for culturing of liver, spleen, and endothelial cells (see Figure 1A). This pump-free multi-organ system incorporated relevant physiological parameters established from previous studies, ensuring the accuracy of physiological flow and shear stress in the platform. The continuous flow design facilitated interactions between organ constructs, ensuring uniform distribution of media, and systematic delivery of therapeutic drugs. Notably, the implementation pump-free microfluidics allowed for the recycling of primary human red blood cells during the creation of this malaria-on-a-chip disease model, enabling the reproduction of systemic infection by the asexual stage of P. falciparum through erythrocytes.
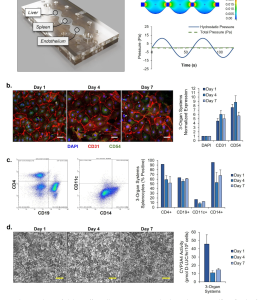 Figure 1. Three-Organ-on-a-Chip System for Housing Functional Human Organ Constructs
Figure 1. Three-Organ-on-a-Chip System for Housing Functional Human Organ Constructs
Characterization of Cells in Multiple Organ Systems
The study initially focused on characterizing cells within the chip. Researchers assessed cell viability, function and morphology at three different time points during the endpoint assay, comparing the findings with those from healthy control systems. Observations were made using phase imaging techniques, immunocytochemical staining, and flow cytometric analysis for endothelial structures, spleen structures, and liver constructs (Fig. 1B–D). In summary, the morphology and viability of cells in the chip model remained stable throughout the testing period, showing no significant decline.
Life Cycle of Plasmodium Falciparum in the Microphysiological System
The study used two strains of P. falciparum: 3D7 (chloroquine-sensitive) and W2 (chloroquine-resistant). Prior to dosing, researchers assessed the parasites’ ability to survive under flow conditions by inoculating multiple organ systems with parasites. Initially, only red blood cells were cultured to characterize parasite viability in the system (Fig. 2A). The platform was inoculated with 3D7 strain, W2 strain, and uninfected red blood cells as controls. Cultures were maintained for 8 days (Fig. 2B), allowing the observation of various stages of the parasite life cycle within the multi-organ platform, including the liver infection stage, the red blood cell cycle, and the sexual reproduction stage. To monitor parasite density levels, samples were collected every 12 hours for blood smear testing (Fig. 2D).
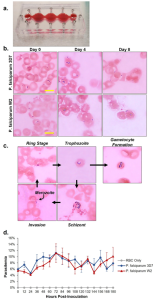 Figure 2. Morphological Progression of Plasmodium Falciparum in the Four-Organ Malaria-on-a-Chip System
Figure 2. Morphological Progression of Plasmodium Falciparum in the Four-Organ Malaria-on-a-Chip System
Vitality of Functional Cells in Microphysiological Systems
The study integrated the single-organ blood model with the three-organ system to create the platform. The system comprised red blood cells infected with the 3D7 strain, W2 strain, or uninfected red blood cells as a control and was cultured for 8 days. Phase imaging was employed to capture functional cells on day 0 before assembly into the system and on day 8 after disassembly(Figure 3A). While the images of various organs were less clear due to co-culture with red blood cells, some morphological changes were observed, indicating that P. falciparum infection disrupted cell viability. Researchers assessed the viability of HUVECs and splenocytes using the Alma blue metabolic reduction method and hepatocytes using the MTT method (Fig. 3B-D). The results revealed no statistical differences revealed between the two systems.
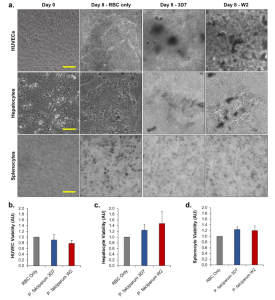 Figure 3. Viability of Functional Cells in the System
Figure 3. Viability of Functional Cells in the System
Adsorption of Chloroquine in the Four Organ Systems
The study began by determining the binding affinity of chloroquine phosphate within the shell before introducing chloroquine into the microbial chip system. A concentration of1000µg/mL (6 mM) chloroquine phosphate was added to the cell-free system, and cultures were collected at time points 0, 4, 8, 12 and 24 hours. Analysis revealed that approximately 50% of the standard starting dose was absorbed within the first 24 hours, indicating a moderate binding affinity of chloroquine to the interior of the system. Based on this discovery, it was estimated that administered doses of 100 µg/mL, 500 µg/mL, and 1000 µg/mL were equivalent to 50 µg/mL, 250 µg/mL, and 500 µg/mL, respectively, after 24 hours of exposure (see Figure 4). Notably, chloroquine was administered as a single dose in this study, with no additional chloroquine was added during medium changes. Considering the prolonged half-life of chloroquine phosphate, a 30% daily decrease in in vitro concentrations was anticipated due to daily media changes.
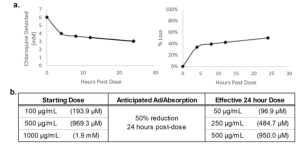 Figure 4. Adsorption/Absorption of Chloroquine in the Cell-Free Malaria Chip System
Figure 4. Adsorption/Absorption of Chloroquine in the Cell-Free Malaria Chip System
Chloroquine Treats Hematocrit and Parasitemia in a Malaria-on-a-Chip System
Chloroquine-sensitive (3D7) and chloroquine-resistant (W2) systems were promptly treated with 100 µg/mL, 500 µg/mL, or 1000 µg/mL of chloroquine upon organ construction. Each system set had a corresponding no-treatment dose control group. Cultures were maintained continuously for 8 days in a flowing environment, with daily replacement of a portion of the medium. Uninfected red blood cells were introduced every 2 days to sustain the hematocrit ratio over the 8-day period (see Fig. 5A, B). Parasite density was monitored every 12 hours. A rapid reduction in parasite density was observed in chloroquine-sensitive systems post-treatment (Fig. 5C). Between 12h and 60h, parasite density was significantly lower than in the untreated control group. However, the decrease was not sustained, with a slight recovery around 72 hours and a complete relapse at 132 hours. In the chloroquine-resistant system (Fig. 5D), while parasite density decreased, the reduction was not as dramatic as in the sensitive system. Parasite density remained significantly lower than the no-treatment control group from 48h to 120h and returned to baseline levels after 132h. Notably, parasite densities in chloroquine-sensitive systems were significantly lower than those in chloroquine-resistant systems between 12h and 60 h.
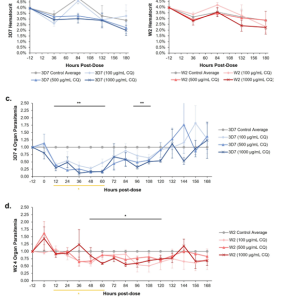 Figure 5. Effect of Chloroquine Dose on Cellular Hematocrit and Parasitemia in Four Organ Systems
Figure 5. Effect of Chloroquine Dose on Cellular Hematocrit and Parasitemia in Four Organ Systems
Viability of Functional Cells in Malaria Chip System After Chloroquine Treatment
Cell viability was assessed immediately after disassembling of each system. For HUVEC cells, a statistically significant and dose-dependent decrease in viability was observed in both systems (see Figure 6A). Spleen cell viability was also assessed, revealing a dose-related decrease, with statistically significant differences observed at higher concentrations in the chloroquine-resistant system (Fig. 6B). Similarly, hepatocytes exhibited a significant decrease in viability with increasing chloroquine concentration (Figure 6C).
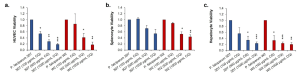 Figure 6. Acute Chloroquine Exposure Negatively Affects the Viability of Functional Cells in a Dose-Dependent Manner
Figure 6. Acute Chloroquine Exposure Negatively Affects the Viability of Functional Cells in a Dose-Dependent Manner
Summary and Discussion
This study showcases the development of a novel platform for studying Plasmodium falciparum, the malaria-causing parasite. Using a serum-free multi-organ system, researchers have created an efficient and cost-effective method to analyze malaria disease mechanisms and evaluate antimalarial drugs. The system allows the efficient observation of disease mechanisms, such as infected red blood cell adhesion to vascular endothelium, immune organ responses to parasitic infection, spleen-mediated clearance of infected cells, and the liver’s response to drug treatment and metabolism. This comprehensive approach aids in antimalarial drug discovery, providing insights into predictable efficacy and off-target effects of novel therapeutic compounds. This study establishes a precedent for future advancements in malaria research, enabling in-depth exploration of parasite interactions with human cells and the study of the both efficacy and off-target effects of emerging therapeutic agents.
Reference:
Rupar MJ, Sasserath T, Smith E, Comiter B, Sriram N, Long CJ, McAleer CW, Hickman JJ. Development of a human malaria-on-a-chip disease model for drug efficacy and off-target toxicity evaluation. Sci Rep. 2023 Jun 28;13(1):10509. doi: 10.1038/s41598-023-35694-4. PMID: 37380653; PMCID: PMC10307889.
Related Services: