2D monolayer cell cultures have long been the main experimental model for drug discovery, while they have limitations for certain applications. For example, they play little role in disease modeling where the cellular microenvironment is important. The study of organoids, spheroids, and 3D cell culture models, has shown great potential in many applications such as disease modeling and regenerative medicine. Therefore, scientists have been working on developing 3D culture systems to better represent real in vivo cellular responses and facilitate in vitro studies of human diseases and personalized medicine.
A research team from the University of California, Irvine, published a review of “Microtechnology-based methods for organoid models” in the journal Microsystems & Nanoengineering, discussing the latest production techniques, current challenges in organoid and spheroid production, and methods for improving microfabrication and microfluidics applications for spheroid and organoid generation.

Organoids and spheroids
Considering morality and research ethics, experiments involving human bodies and human embryonic tissues are greatly restricted. Although human cell monolayer culture can be used for certain biological studies, 2D cell culture cannot reflect the complex structure and microenvironment of in vivo tissues. As technology advances, three-dimensional human-like tissues, known as organoids and spheroid models, have become popular objects of study. Typically, spheroids are formed from cancer cell lines or clusters of dissociated cells from tumor tissue in a non-adherent stroma (Figure 1a). Most organoid models use stem cells (ESCs or iPSCs) as the cell source (Figure 1b).
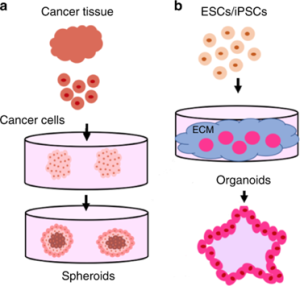 Figure 1. Differences in the origin of spheroids and organoids
Figure 1. Differences in the origin of spheroids and organoids
Techniques for Organoids and Spheroids Production
1. ECM scaffold method
Extracellular matrix (EMC) scaffolding is one of the most commonly used techniques in organoid production. Adult stem cells are first coated on a commonly used ECM protein mixture gel matrix and maintained under culture conditions. When specific cell types are desired, cells are then stained with antibodies, sorted using a fluorescence-activated cell sorting (FACS) system, and plated on individual gel matrix dishes (Figure 2). The advantage of this method is that it can monitor cell biological processes (such as cell adhesion, migration, and chemotaxis) in a tissue-like environment. The disadvantage is low reproducibility because different hydrogel production batches have different mechanical properties, which can affect the formation of organic matter.
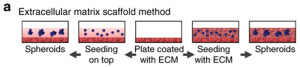 Figure 2. Schematic diagram of the steps of the ECM scaffold method
Figure 2. Schematic diagram of the steps of the ECM scaffold method
Figure 3 shows examples of liver organoids generated by the ECM scaffolding method. The resulting liver organoid model was over 1 mm in diameter, but live cell staining measurements showed minimal cell death in this model. Furthermore, by quantitative RT-PCR, it was found that liver-derived ECM hydrogel (LEMgel) could enhance the expression of markers for promoting the maturation of hepatocytes to a level closer to that of the human liver.
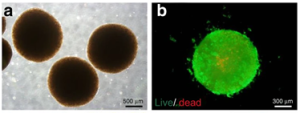 Figure 3. Liver Organoids Generated by the ECM Scaffold Method
Figure 3. Liver Organoids Generated by the ECM Scaffold Method
2. Rotating Bioreactor Method
This method mainly uses the principle of suspension culture and uses stirring to prevent cells from attaching to the surface of the culture dish (Figure 4). Disadvantages of this method are the production of spheroids with widely varying sizes, and the potential for shear forces experienced by cells in the bioreactor to affect cell physiology.
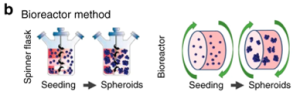 Figure 4. Schematic diagram of the rotating bioreactor method
Figure 4. Schematic diagram of the rotating bioreactor method
As shown in Figure 5, kidney organoids were mass-produced using this method, and organoid models larger than 700 μm in diameter showed increased apoptosis, suggesting that controlling organoid size in 3D culture is critical for improving cell viability.
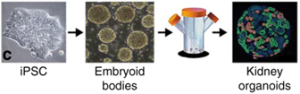 Figure 5. Kidney organoids produced by the spinning bioreactor method
Figure 5. Kidney organoids produced by the spinning bioreactor method
3. Hanging drop method
The method is based on the aggregation of cells at the liquid-air interface to form spheroids. Cells are first suspended in a drop of culture medium and placed inside the lid of a Petri dish. The droplets are simultaneously subjected to surface tension and gravity, and the lid with the suspended cells is placed in a Petri dish to grow spheroids (Figure 6). The advantages of this method are simplicity, no need for special substrates, high product consistency, and high-throughput production; while the disadvantages are the high cost of liquid handling robotics, limited droplet size, and inability to replace medium midway.
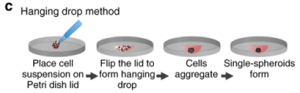 Figure 6. Schematic diagram of the pendant drop method
Figure 6. Schematic diagram of the pendant drop method
4. Low adherent cell culture plate method
The principle of the method is to use a low-adhesion or hydrophilic treated cell culture plate to allow cultured cells to clump and form spheroids (Figure 7). This method usually obtains spheroids with a diameter of 370-400 µm, which has the advantages of easy operation, high-throughput production, and cost efficiency, but the disadvantage is that it is not suitable for some specific cell types and requires additional experimental steps.
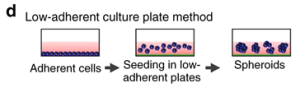 Figure 7. Schematic diagram of low adherence cell culture plate method
Figure 7. Schematic diagram of low adherence cell culture plate method
5. Magnetic levitation method
The method involves first incubating cells overnight with phage, polydisperse gold nanoparticles, and magnetic hydrogel-coated iron oxide nanoparticles. The nanoparticles are then taken up by the cells and digested with trypsin., then they are placed on a low-attachment plate with the magnetic lid placed on top to attract the nanoparticles and generate a liquid-air cell suspension (Figure 8). The advantages of this method are fast growth of spheroids, no need for special substrates, and reproducible necrotic and hypoxic regions, while the disadvantages are that nanoparticles are expensive and potentially cytotoxic.
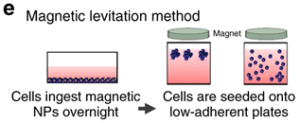 Figure 8. Schematic diagram of the magnetic levitation method
Figure 8. Schematic diagram of the magnetic levitation method
6. Bioprinting method
The principle of this method is derived from 3D printing technology, which uses a bioprinter to accumulate biological materials layer by layer, and finally achieves the required complex structure. The advantage of this technology is that it can achieve accurate and versatile printing of multiple components, which can be customized, but the disadvantage is that bio-inks with the required properties and viscosity are difficult to obtain.
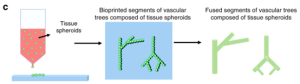 Figure 9. Schematic diagram of the bioprinting method
Figure 9. Schematic diagram of the bioprinting method
Challenges in organoid and spheroid production
1. Difficult to reconstruct the complex environment in vivo
Although organoids and spheroids can establish a certain microenvironment, they lack the reproduction of the real environment in vivo such as immune mechanical stimulation and contact with other cells.
2. Difficult to deliver nutrition and gas
For example, brain organoid models can take weeks to form fully mature and functioning neurons, while the long-term maturation and growth of these 3D brain models prevent efficient nutrients, gas, and waste exchange, mainly because of the lack of proper angiogenesis.
3. Non-reproducible in vitro models
This shortcoming of lacking reproducible and standardized models is also mentioned in several of the methods described above. And it is difficult to control the size and cell number of these models without considering the physical and scaffold geometry constraints.
Microfabrication-based solutions in organoids and spheroids
1. Micropatterning
Micropatterning methods include techniques such as microcontact printing and soft lithography. The principle of microcontact printing is shown in Figure 10. First, a PMMA plate was micromilled to form a mold for a PDMS stamper, and another PMMA plate was ground to form wells with connected flow channels, then a series of RGD peptide binding sites were created in the center of the wells with a PDMS stamp. The primary cells are seeded into the wells, and the entire assembly (also known as a spheroid microarray chip) was dipped in ethanol containing 5 mM PEG-SH to eliminate nonspecific cell binding around the RGD peptide region, resulting in spheroids. The obvious advantage of this technology is that it can be produced in high throughput. The disadvantage is that if manual printing is used, the reproducibility and pattern formation efficiency are not high.
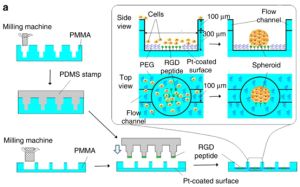 Figure 10. Schematic diagram of micropatterning technology
Figure 10. Schematic diagram of micropatterning technology
Soft lithography, as another micropatterning method, besides enabling mass production, is more precise and reproducible than manual microcontact printing. However, this technology has high requirements on equipment and experimental environment, and the steps are cumbersome and expensive. The reproducibility of 3D cell culture is significantly enhanced by the geometric confinement of micropatterning.
2. Microfluidics
The biggest impact of microfluidics on 3D culture is that it can continuously inject nutrients and growth factors. Matrigel allows proper dispersion of nutrients, gases, and soluble substances, avoiding cell necrosis and promoting stem cell differentiation. Figure 11 shows the process of generating brain organoids using microfluidics. The embryoid body is encapsulated with matrigel, and the medium is perfused through the adjacent channel to promote the differentiation of the embryoid body to form a brain organoid. This assembly is also called a microfluidic organ chip. Microfluidics can be used for high-throughput production at a lower cost than conventional techniques, with the disadvantage of lower numbers of usable cells.
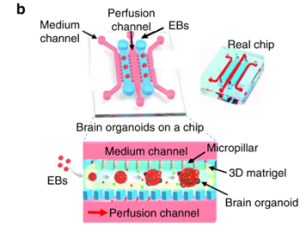 Figure 11. Schematic diagram of microfluidic technology to generate brain organoids
Figure 11. Schematic diagram of microfluidic technology to generate brain organoids
Future direction
Although microtechnology solutions have overcome many of the limitations of traditional 3D culture, they still have great potential for development, for example, integrating multiple organoids from different tissues into a single platform to achieve a complex microenvironment closer to the real body, or assembling to form human functional units to realize the correlation of human tissue in vitro. Continuous advances in future microtechnology have significant implications for drug development, disease modeling, and diagnostic research.
Reference:
Velasco, V., et al. Microtechnology-based methods for organoid models. Microsyst Nanoeng 6, 76 (2020). https://doi.org/10.1038/s41378-020-00185-3
Related Services: