On October 31, 2023, the research team from Eindhoven University of Technology released a review paper titled “Cancer-on-chip models for metastasis: importance of the tumor microenvironment” in the journal Trends in Biotechnology. This paper comprehensively examines recent advancements in Cancer-on-a-Chip (CoC) models, with a particular focus on three pivotal components of the Tumor Microenvironment (TME): (i) the anisotropic extracellular matrix (ECM) structure, (ii) the vasculature, and (iii) the immune system. The primary objective of this article is to offer guidance to biological researchers in selecting the most suitable CoC approach for addressing inquiries concerning the role of the TME in metastasis, while also inspiring engineers to innovate and create novel CoC technologies.

The Imperative for Cancer-on-a- Chip Models
The demise of patients due to cancer seldom results from the primary tumor; rather, it is the secondary tumors originating from metastases that lead to cancer progression and mortality. Metastasis encompasses at least five sequential stages: (i) invasion, where cancer cells escape from the primary tumor by traversing the basement membrane and stromal tissue, (ii) penetration of the blood vessel wall and entry into the blood circulation, (iii) survival of cancer cells in the blood circulation, (iv) extravasation of cancer cells and seeding in distant organs, and (v) the subsequent growth of tumors at metastatic sites (see Figure 1). Extensive studies affirm the pivotal role played by the TME in each of these stages. As shown in Figure 1, the TME is a dynamic and complex structure composed of multiple components such as ECM, blood vessels, lymphatics, immune cells, and stromal cells. Unlike healthy tissue, the TME in many tumors is characterized by increased extracellular matrix stiffening and alignment, vascular irregularities/leakage, tumor-associated inflammation, and inhibited tumor cell killing.
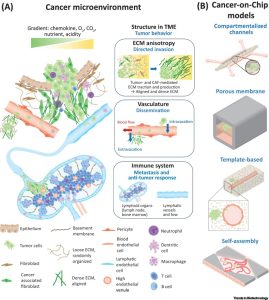 Figure 1. Schematic representation of the tumor microenvironment (TME) and different types of cancer-on-a-chip (CoC) models
Figure 1. Schematic representation of the tumor microenvironment (TME) and different types of cancer-on-a-chip (CoC) models
To dissect the individual contributions of TME components to metastasis in a controlled manner, three-dimensional multicellular analysis within a controlled TME is essential. Recently, CoC models have emerged as valuable tools for studying different stages of tumor progression, forming a subset of Organ-on-a-Chip (OoC) models. The CoC model is based on a microfluidic chip containing a tumor cell culture chamber, which allows control over the cellular composition, local gradients, fluid flow, and tissue mechanics within the TME. Figure 1 illustrates the four main categories of OoC models, namely: compartmental channel models, porous membrane models, template-based models, and self-assembly models.
ECM in Cancer Chip Models
In healthy organisms, epithelial cells rest on a basement membrane composed of type IV collagen and laminin, beneath an interstitial tissue rich in type I collagen, elastin, and proteoglycans. This collagen-rich ECM exhibits distinctive microanatomical structures that facilitate immune cell influx, inflammatory responses, and the diffusion of oxygen and nutrients. In the TME, due to malignant transformation, cells disintegrate the basement membrane, invade, and remodel adjacent stromal tissue (see Fig. 1). Prior to and conurrently with invasion, cancer-associated fibroblasts (CAFs) may deposit fibronectin, type I or type IV collagen, or proteoglycans, resulting in the stiffening of ECM-rich fibrous connective tissue. The stiffened ECM leads to increased mechanical coupling mediated by integrin signaling and actomyosin contraction. Consequently, cellular traction forces deform the network from an initially curved and relaxed structure to a stretched and linearized structure, promoting local cancer invasion by enhancing integrin signaling and ultimately leading to higher tumor incidence. Therefore, the design of CoC models should carefully consider the impact of physicochemical changes in ECM structure and stiffness on tumor development. Recent research has focused on designing anisotropic matrix structures, specifically type I collagen, with the goal of reproducing the well-aligned, in vivo structure of collagen through (i) engineering, (ii) directional force derivation, or (iii) cell derivation. Interaction of collagen-rich ECM with invasive tumors (Fig. 2A-C).
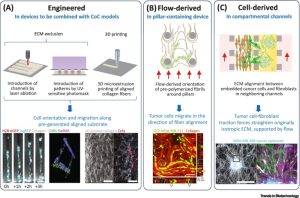 Figure 2. Methods to obtain anisotropic extracellular matrix (ECM) architecture in cancer-on-a-chip (CoC) models
Figure 2. Methods to obtain anisotropic extracellular matrix (ECM) architecture in cancer-on-a-chip (CoC) models
The engineering approach considers controlled ECM architecture for tumor growth and invasion. Altering the hydrogel concentration, polymerization conditions, or glycation allows the creation of hydrogels with anisotropic structures that guide tumor invasion. Techniques like electrospinning or 3D microextrusion printing can produce highly aligned collagen sheets or hydrogels. These engineering methods, potent in ECM alignment, hold potential for integration with CoC models. The orientation of ECM can be achieved by applying directional forces, such as external strain, flow, or shear, during or after fiber polymerization. CoC models, are designed to facilitate fluid flow, benefit from the anisotropy of hydrogels generated by flow. Three-dimensional cell placement is attainable by preforming anisotropic networks and introducing cells post-polymerization, or by embedding cells in an ECM solution before polymerization. While there might be less spatial control between fibrils compared to engineered ECM patterning methods, fibril spacing can be quantified through image analysis. In conclusion, the generation of flow-induced anisotropy presents significant application potential for CoC devices, and its combination with soluble serum or chemokine gradients, interstitial flow, or perfusable microvasculature holds promise.
Vascular System in CoC Models
The examination of cancer-blood vessel interactions is facilitated by the vascularized CoC model. A compartmental channel model of angiogenesis in cancer spheroids enables the study of tumor perfusion and drug delivery (see Figure 3A). Fibroblasts within tumor spheroids triggers sprouting of side channel endothelium toward the central compartment containing the spheroids, forming a microvascular network that perfuses and connects to the spheroids. A template-based model establishes primary mammary ducts and microvascular endothelium, and the paracrine crosstalk between them was studied. The model consisted of a perfused endothelialized vascular lumen embedded in ECM with an adjacent vertically oriented second closed end lumen seeded with human mammary epithelial cells (see Fig. 3B). In a self-assembly model of vascularized human colorectal cancer, irregular, leaky, and poorly perfused microvasculature, typical of cancer, is observed (see Figure 3C). These examples demonstrate that CoC models effectively simulate and study the interactions between tumors and vasculature in a controlled manner.
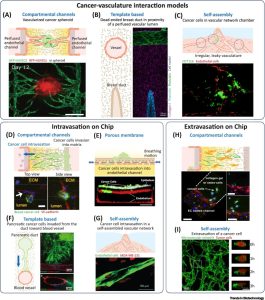 Figure 3. Vascular system in cancer-on-a-chip (CoC) model
Figure 3. Vascular system in cancer-on-a-chip (CoC) model
Immune System in CoC Models
Immune cells in the TME play a crucial role not only in killing tumor cells but also in supporting their metastatic behavior. Soluble factors secreted by cancer cells attract monocytes to tissues, where they differentiate into M1 and M2 macrophage subpopulations with antitumor or protumorigenic functions. TNF-α secreted by macrophages increases endothelial barrier permeability, facilitating intravasation of tumor cells. Monocytes and macrophages are also involved in tumor cell extravasation. In compartmental channel devices, inflammatory monocytes, disrupt endothelial tight junctions, invade the surrounding hydrogel, and differentiate into macrophages, promoting tumor cell extravasation and invasion through microchannels. Similar results are observed in more complex CoC models, with the formation of a microvascular network within the hydrogel (Fig. 4). CoC models prove valuable for studying the role of immune cells in tumor cell metastasis and exploring therapeutic inhibitors and blocking antibodies that modulate the migration and adhesion of immune and tumor cells during tumor cell extravasation.
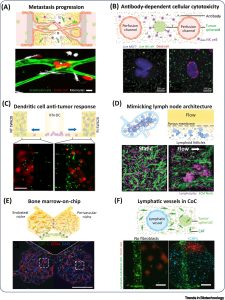 Figure 4. Immune system in cancer-on-a-chip (CoC) model
Figure 4. Immune system in cancer-on-a-chip (CoC) model
Summary and Discussion
The use of CoC models to study various stages of tumor progression, especially metastasis, has seen rapid growth. These models offer insights into the effects of specific TME components on tumor behavior and can be applied to study the impacts of cancer treatments , including chemotherapy, nanoparticles, immunotherapy, and ECM-targeted strategies. As CoC models become more realistic, their applications expand. A promising avenue is combining CoC models with clinical materials for studying patient-specific cancer progression, potentially transforming drug efficacy testing and the development of new treatments to prevent metastasis.
Reference:
Jouybar M, de Winde CM, Wolf K, Friedl P, Mebius RE, den Toonder JMJ. Cancer-on-chip models for metastasis: importance of the tumor microenvironment [published online ahead of print, 2023 Oct 30]. Trends Biotechnol. 2023;S0167-7799(23)00292-5. doi:10.1016/j.tibtech.2023.10.001
Related Services: