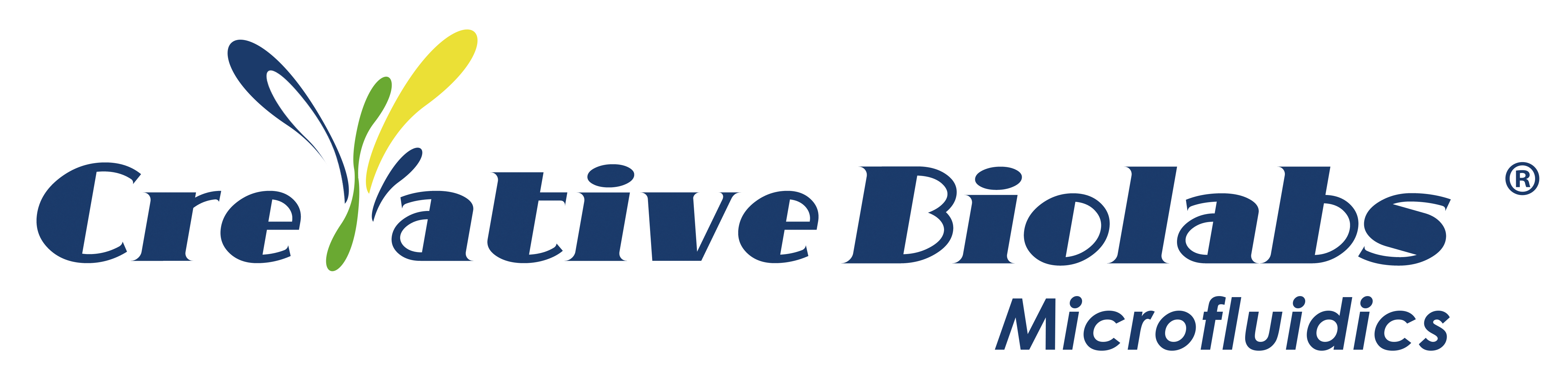The liver is the largest internal organ in the human body and plays a leading role in maintaining normal physiological activities, such as controlling blood sugar and ammonia levels, synthesizing various hormones, detoxifying endogenous, and exogenous substances. Normally, the liver has an extremely strong regenerative capacity in response to physical and chemical damage. However, damage caused by adverse reactions to drugs and chronic diseases can permanently impair their ability to perform physiological functions. Before entering clinical trials in humans, new drug candidates must be evaluated for safety in animal models, usually rats and dogs. However, these models do not always correctly predict drug toxicity in humans, especially toxicity related to drug-induced liver injury (DILI), or results from different models may be inconsistent. This situation leads to the failure of drugs in trials and clinical trials, as well as the huge cost of drug development, and there is an urgent need to propose better predictive alternatives.
As a solution, organs-on-a-chip (OoC) reconstructs the 3D microenvironment and function of organs. It has become an alternative candidate for cell experiments and drug screening. The liver chip platform recapitulates human liver physiology and pathology by recreating liver architecture, maintaining high cell viability and cell phenotype, and simulating native liver function. Therefore, it is usually more predictive of drug candidate outcomes in humans.
 Schematic diagram of the liver organ chip model
Schematic diagram of the liver organ chip model
Characteristics of the cells used in the liver-on-a-chip
(1) Primary hepatocytes
Advantages: Inclusion of intrinsic features of the liver, including phase I and phase II metabolic enzyme activity, glucose metabolism, and ammonia detoxification.
Limitations: Loss of liver-specific functions, not suitable for long-term use, high cost, donor variability, and difficulty to separate.
(2) Liver-derived cell lines
Pros: Unlimited lifetime, easy manipulation, phenotypically stable, and critical for drug metabolism and toxicity.
Limitations: Inaccurate drug response, low metabolic capacity, and rapid loss of expression of liver-specific enzymes/transporters.
(3) Stem cells induce hepatocytes
Advantages: Stable source of hepatocytes, liver organoids, and stable functions, including albumin secretion, liver-specific gene expression, urea production, and metabolic activity.
Limitations: Difficult to operate, requirement of specific inducer, high cost, and not mature enough.
Liver organ chip construction technology
2D planar culture-based liver-on-a-chip
2D planar cultures are widely used in the early stages of drug screening because they are easy to handle and suitable for screening large numbers of compounds in a short period of time. However, accumulating evidence suggests that 2D planar culture of hepatocytes results in rapid loss of expression and phenotype of liver markers within hours, meaning it is not suitable for long-term culture, and also, the interactions between hepatocytes and non-hepatocytes are very weak. To mimic the complexity of the interaction of each cell type in the liver, the researchers developed methods to pattern and co-culture hepatocytes with other cell types on 2D matrices. The micropatterned matrix not only alters the distribution of different cell types in a controllable manner, but also provides suitable biochemical cues for parenchymal and non-parenchymal cells. Researchers found that the surface characteristics of 2D materials, such as stiffness and hydrophilicity, also affect the phenotype and function of liver cells. Therefore, developing suitable materials has become the development direction of 2D planar culture.
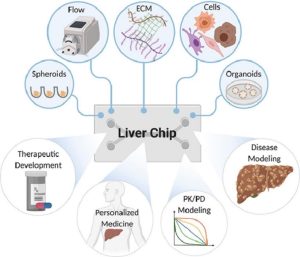
Liver-on-a-chip based on matrix-dependent 3D culture
In liver tissue engineering, an extracellular matrix (ECM)-like scaffold is needed to promote cell adhesion, support cell growth, and enhance cell-matrix interactions. Therefore, various ECM components (natural and synthetic) have been applied in liver-on-a-chip to improve liver function for drug and cytotoxicity studies. The researchers used the ECM in a liver-on-a-chip to maintain and mimic a native microenvironment. Although hydrogel scaffolds have structural and mechanical properties similar to ECMs, artificial 3D systems still lack proper natural growth factors to promote cell growth and maintain cellular functions. Therefore, decellularized liver matrix (DLM) has become the most promising candidate for engineering native liver tissue. A bionic 3D liver tumor chip has been developed using DLM and gelatin metharcyloyl (GelMA) in a microfluidic 3D dynamic cell culture system, which better reproduced the tumor microenvironment, such as basic scaffold proteins, growth factors, hardness, and shear stress.
 Strategies for constructing liver-on-a-chip in matrix-dependent 3D culture
Strategies for constructing liver-on-a-chip in matrix-dependent 3D culture
Liver-on-a-chip culture based on matrix-free 3D spheroids
Aggregating hepatocytes into 3D spheroids is another routine approach and a promising in vitro model for the study of liver metabolism and cytotoxicity. Methods for culturing substrate-free 3D spheroids include the hanging drop technique, the cell exclusion plate method, and the microwell array method. The hanging drop technique facilitates cell aggregation, resulting in spheroids, which can be maintained for more than 9 days in the formation of primary human liver microtissues using hanging hydrogel drops. The cell exclusion plate method is commonly used to self-assemble hepatocyte spheroids. The researchers modified primary human hepatocytes with magnetic nanoparticles and then applied magnetic force to quickly assemble and easily obtain spheroids. Furthermore, in toxicology applications, the metabolic parameters and cell viability of individual spheroids can be monitored without a microscope by integrating the microsensor with the liver-on-a-chip. Microwell arrays can also be applied to generate 3D spheroids in a high-throughput and controllable manner, the size of which depends on the size of the microwells.
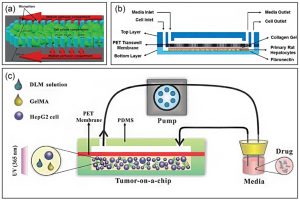
Layer-by-layer deposition-based liver-on-a-chip
The liver contains many functionally repeating microvascular units with unique structural features. This layered structure allows the construction of a liver-on-a-chip by layer-by-layer deposition. For example, a layer-by-layer deposition method was designed to sequentially seed hepatocytes, endothelial cells, and stellate cells on hollow fiber membranes. Cells attach to the fiber surface, self-assemble, form tissue-like structures around and between fibers, and synthesize albumin and urea within 28 days. Due to the flexibility of microfabrication and microfluidics, multichannel microfluidic chips have emerged as a promising platform to reproduce key tissue features of the liver. For example, the collagen loaded with the liver-derived cell line HepG2 cells and human umbilical vein endothelial cells HUVEC were injected into the microfluidic chip synchronously, and then taking advantage of the laminar flow, the two layers of collagen would form a clear boundary, by controlling the cell density and Upon injection of growth factors, HUVECs in collagen can self-assemble into monolayers.
 Liver-on-a-chip built using layer-by-layer deposition
Liver-on-a-chip built using layer-by-layer deposition
3D bioprinted liver-on-a-chip
3D bioprinting mainly uses cells, biological hormones, growth factors, intercellular substances, and other substances to print out living tissues with biological functions. 3D bioprinting can produce anatomically accurate liver structures, including the specific spatial structure and vascular network of the liver. The unique features of 3D bioprinting technology make it an increasingly popular tool for fabricating in vitro liver models. For example, the researchers used bioprinting to create a human liver model containing primary hepatocytes, hepatic stellate cells, and endothelial cells to simulate fibrosis following methotrexate- and thioacetamide-induced liver injury. After exposure to these compounds, the model was detected with liver damage, including hepatocellular damage, as well as deposition and accumulation of fibrillar collagen, suggesting that the 3D bioprinted liver reproduced the compound-induced liver injury response. However, 3D liver analogs printed in conventional flat sheets or transwells cannot provide dynamic perfusion conditions. Therefore, further combining the bioprinted liver with microfluidic and perfusion devices to form a liver-on-a-chip can further enhance liver function.
Application of Liver Organ Chip Model
Drug Screening and Toxicity Testing
Drug-induced liver injury remains a significant cause of restrictive drug labeling and post-market withdrawal of therapeutics. Liver organ-on-a-chip can simulate physiological parameters in vivo to assess the behavioral response of cells to drugs, which provides an alternative to animal experiments. For example, the researchers designed a perfused incubator liver-on-a-chip cultured with 3D spheroids that maintained cell viability for more than 24 days. Next, chronic drug responses to repeated doses of diclofenac and acetaminophen were assessed by the device.
Metabolic prediction
One of the goals of establishing a liver-on-a-chip is to establish the same hepatocyte microenvironment in vitro as in vivo. Compared with 2D cell culture, the comprehensive metabolic capacity of hepatocytes cultured on organ chips was greatly enhanced. In addition, the liver-on-a-chip provides a dynamic flow environment for hepatocytes that allows for higher albumin synthesis and urea excretion compared with static culture. Another important function of the liver is to participate in drug metabolism. The Liver Organ Chip can be used to assess phase I and II metabolisms in the liver and to study the first-pass metabolism of drugs by integrating a gut-like structure on the front of the liver chip.
Model liver disease
Based on the fact that organ chips can control the characteristics of the external microenvironment of cells, a series of liver disease models can be established, including alcoholic liver disease, fatty liver, liver fibrosis, acute liver injury, and patient-specific liver diseases. For example, in Lee’s study, rat primary hepatocytes and HSCs were co-cultured on a fluidic active chip to observe structural changes, and liver function gradually decreased with increasing ethanol concentration. In addition, liver-on-a-chip devices have also been used to study host/pathogen interactions. A 3D microfluidic liver culture system has been constructed, which provides a valuable preclinical platform for hepatitis B virus (HBV) research, which can reproduce all steps of the HBV life cycle, including patient origin Replication of HBV and maintenance of HBV cccDNA [10]. In these examples, the liver-on-a-chip provides advanced disease modeling with better preservation of the liver phenotype, which is critical to facilitate the development of precision medicine and targeted drugs.
Expansion of multi-organ-on-a-chip models
Physiological processes including drug hepatotoxic metabolism are also involved in other organs besides the liver. Therefore, organ-on-a-chip should also consider the interactions between multiple organs to study complex mechanisms in diseases and drug screening. At present, a series of multi-organ chips have been established to simulate the physiological environment system of multiple organs, such as intestine and liver, lung and liver, and even four-organ joint chips of the intestine, liver, skin, and kidney (pictured below). For example, to evaluate the interaction of nanoparticles with human tissues, a gastrointestinal and liver cell system was constructed by co-culturing intestinal epithelial cells (Caco-2) with mucin-secreting cells (TH29-MTX) and HepG2/C3A cells. High doses of nanoparticles induce aspartate aminotransferase release, as an indicative of hepatocellular injury. Thus, the device successfully mimics in vivo acetaminophen uptake, metabolism, and toxicity in vitro.
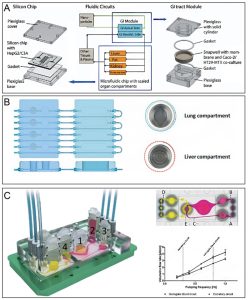 Schematic diagram of various multi-organ chip systems
Schematic diagram of various multi-organ chip systems
Reference:
[1] Moradi E; et al. Microfluidic organ-on-a-chip models of human liver tissue[J]. ACTA BIOMATER, 2020, 116: 67-83.
[2] Yajima Y; et al. Development of a perfusable 3D liver cell cultivation system via bundling-up assembly of cell-laden microfibers[J]. J Biosci Bioeng, 2018, 126: 111–118.
[3] Deng J; et al. Engineered Liver-On-A-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review[J]. Micromachines (Basel), 2019, 10(10): 676.
[4] Aeby E A; et al. Microfluidic Hydrogel Hanging-Drop Network for Long-Term Culturing of 3D Microtissues and Simultaneous High-Resolution Imaging[J]. Adv Biosyst, 2018, 2: 1800054.
[5] Ahmed H M M; et al.3D liver membrane system by co-culturing human hepatocytes, sinusoidal endothelial and stellate cells[J]. Biofabrication, 2017, 9: 25022.
[6] Norona L M; et al. Editor’s Highlight: Modeling Compound-Induced Fibrogenesis In Vitro Using Three-Dimensional Bioprinted Human Liver Tissues[J]. Toxicol Sci, 2016, 154(2): 354-367.
[7] Yu F; et al. A perfusion incubator liver chip for 3D cell culture with application on chronic hepatotoxicity testing[J]. Sci Rep, 2017, 7: 14528.
[8] Choe A; et al. Microfluidic Gut-liver chip for reproducing the first pass metabolism[J]. Biomed Microdevices. 2017, 19: 4.
[9] Lee J; et al. A 3D alcoholic liver disease model on a chip[J]. Integr Biol, 2016, 8: 302–308.
[10] Ortega-Prieto A M; et al.3D microfluidic liver cultures as a physiological preclinical tool for hepatitis B virus infection[J]. Nat Commun, 2018, 9: 682.
[11] Esch M B; et al. Body-on-a-chip simulation with gastrointestinal tract and liver tissues suggests that ingested nanoparticles have the potential to cause liver injury[J]. Lab Chip, 2014, 14: 3081–3092.
Related Services: