In recent years, adoptive cell therapy (ACT) has demonstrated immense potential in cancer treatment. The remarkable efficacy of CAR-T cells in leukemia therapy is well recognized. Meanwhile, the FDA’s approval of two cell therapies for solid tumors in 2024 marks a new phase in the field. However, ACT’s large-scale production remains challenging due to its reliance on patient-derived live cells. The current centralized manufacturing model faces limitations in clinical application, including complex production processes, significant cell heterogeneity, high costs, and inconsistent efficacy. Therefore, developing innovative manufacturing equipment and processes to facilitate the large-scale clinical application of cell therapy has become an urgent priority.
A research team recently published a review article in Nature Biomedical Engineering titled “Microfluidic technologies for enhancing the potency, predictability, and affordability of adoptive cell therapies.” This paper systematically examines the transformative role of microfluidics in various stages of ACT cell preparation.
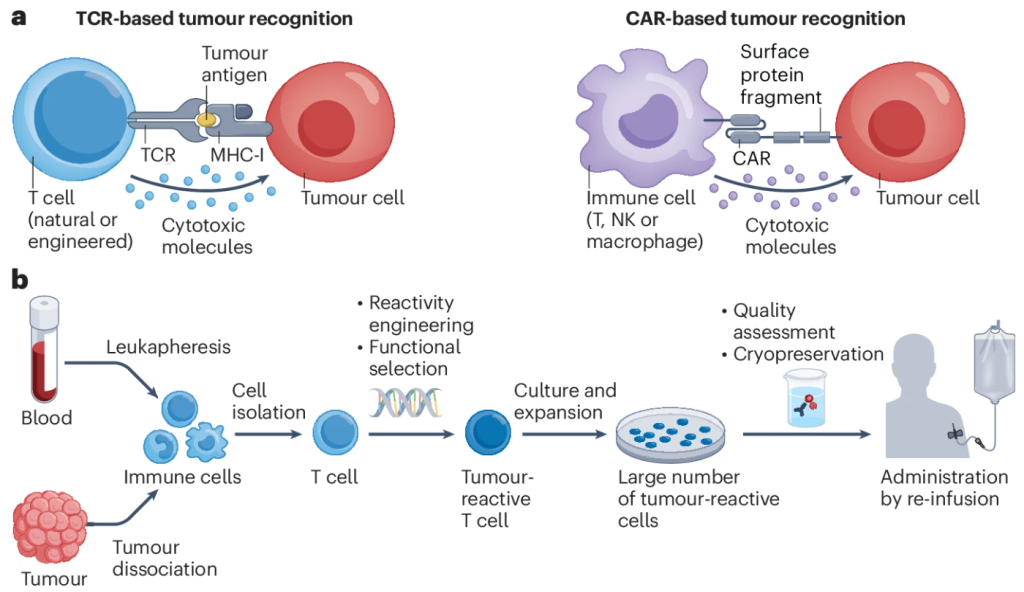 Figure 1. Cellular Therapies for Cancer Treatment
Figure 1. Cellular Therapies for Cancer Treatment
Pain Points of ACT Therapy: Why Has It Not Been Widely Adopted?
Despite CAR-T cell therapy achieving a complete remission rate of over 50% in cancers like B-cell lymphoma, its large-scale adoption remains hindered by three key challenges:
- Prolonged Manufacturing Timeline
Current ACT therapies involve multiple critical steps, including cell enrichment, genetic modification, expansion, and quality control, with the entire process taking 15–33 days. For rapidly progressing cancers such as acute leukemia, this extended waiting period may cause patients to miss the optimal treatment window.
- High Functional Heterogeneity of Cells
Expanded ACT cells exhibit significant variability across patients. In some cases, the cells proliferate efficiently and exert strong anti-cancer effects, while in others, they fail to expand, leading to treatment failure. This unpredictability severely impacts the stability and generalizability of ACT therapy.
- Exorbitant Manufacturing Costs
The costs associated with cell enrichment, genetic editing, and large-scale expansion are prohibitively high. Leading pharmaceutical companies price a single ACT therapy dose at $370,000–500,000, while even domestically developed alternatives cost hundreds of thousands of RMB, making treatment financially burdensome for most patients.
These challenges significantly limit the widespread adoption of ACT therapies. Breakthrough technologies are urgently needed to enhance manufacturing efficiency, reduce costs, and improve the predictability of therapeutic efficacy.
How Can Microfluidic Technology Overcome ACT Manufacturing Bottlenecks?
The core advantage of microfluidic technology lies in its ability to precisely control micron-scale fluids and single cells, thereby optimizing key steps in ACT production. Currently, microfluidics has achieved significant advancements in four critical stages of ACT preparation.
- Cell Enrichment
Traditional fluorescence-activated cell sorting (FACS) and magnetic bead-based sorting (MACS) have low efficiency in large-scale cell enrichment, with limited post-sorting cell viability. In contrast, next-generation magnetic microfluidic sorting significantly enhances enrichment efficiency (Figure 2).
For instance, BioArt’s previously reported MATIC technology has achieved a high-throughput enrichment rate of 320 million cells per hour, providing an efficient solution for obtaining immune cells required for therapy.
Additionally, microfluidic technology can precisely measure and control biomechanical forces at the nanonewton (nN) to piconewton (pN) scale, allowing the selection of optimal CAR and TCR immune cell clones. This capability improves affinity selection, enhancing both the treatment predictability and anti-cancer potency of ACT cells.
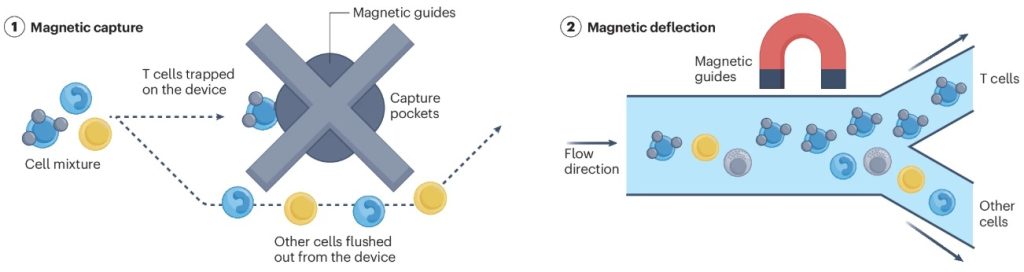 Figure 2. Working Principle of Magnetic Microfluidic Sorting Technology
Figure 2. Working Principle of Magnetic Microfluidic Sorting Technology
- Gene Editing
Currently, gene editing in ACT cells primarily relies on viral vectors (e.g., lentivirus and retrovirus). However, these methods involve high production costs and pose potential risks of insertional mutations.
In contrast, microfluidic transfection technologies (such as mechanoporation and electroporation) temporarily alter cell membrane permeability within minutes (Figure 3), enabling non-viral gene delivery with up to 90% transfection efficiency.
This approach significantly enhances efficiency, safety, and cost-effectiveness in gene editing, providing a more advantageous solution for large-scale ACT cell production.
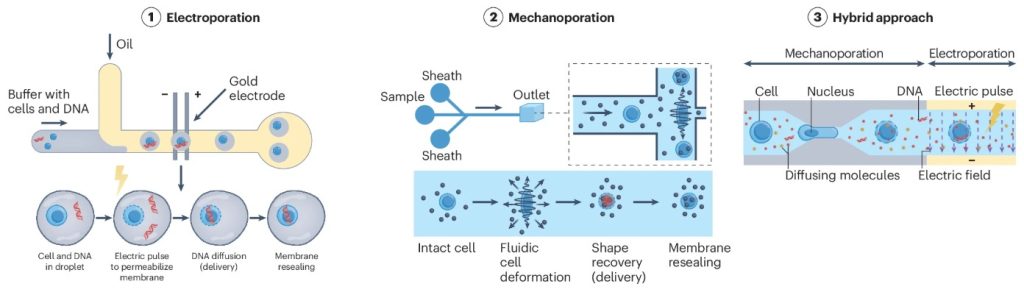 Figure 3. Working Principle of Microfluidic Transfection Technology
Figure 3. Working Principle of Microfluidic Transfection Technology
- Cell Culture
In traditional bioreactors, expanding immune cells typically requires 1–10 liters of culture medium and large amounts of cytokines, further driving up ACT therapy production costs.
Microfluidic bioreactors precisely regulate the cell culture environment, enabling high-density expansion at 150 × 10⁶ cells/mL. Moreover, microfluidic systems can continuously monitor key parameters such as oxygen levels, pH, and nutrient concentrations, dynamically adjusting medium exchange to ensure optimal cell growth (Figure 4).
Compared to conventional methods, microfluidic bioreactors significantly reduce expansion costs, improve expansion speed, and offer an innovative solution for enhancing the efficiency and affordability of ACT therapy.
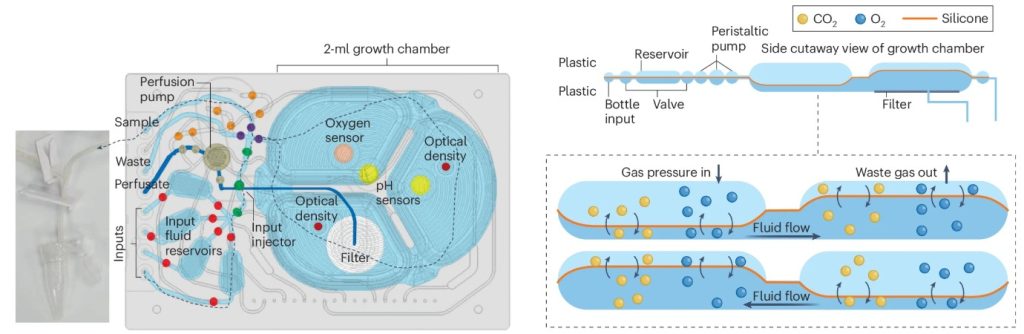 Figure 4. Schematic Diagram of a Microfluidic Bioreactor
Figure 4. Schematic Diagram of a Microfluidic Bioreactor
- Efficacy Prediction
Traditional ACT evaluation relies on simple 2D co-culture systems to assess the therapeutic potency of manufactured cells. However, this approach is widely recognized as inadequate for accurately predicting in vivo efficacy.
Microfluidic systems (e.g., organ-on-a-chip) can reconstruct a highly biomimetic 3D microenvironment, providing immune cells with more physiologically relevant conditions. By introducing manufactured immune cells into an organ-on-a-chip system, researchers can more accurately simulate their in vivo behavior and potency, enhancing treatment predictability.
This technology optimizes ACT manufacturing processes and ensures that final cell products exhibit robust therapeutic effects in patients.
 Figure 5. Microphysiological System for Evaluating and Predicting Therapeutic Efficacy
Figure 5. Microphysiological System for Evaluating and Predicting Therapeutic Efficacy
Conclusion and Outlook
Microfluidic technology, with its superior performance in centimeter- and millimeter-scale devices, is driving a revolutionary transformation in the preparation and optimization of cell therapies, surpassing the capabilities of traditional instruments. Several microfluidics-based cell therapies have already entered Phase I/II clinical trials, such as Miltenyi Biotech’s MACSQuant Tyto and CTRL Therapeutics’ IsoQore.
As microfluidic technology continues to mature and commercialize, several key advancements can be anticipated:
- Decentralized, point-of-care cell therapy manufacturing will become a reality, enabling most patients to receive personalized treatments within days or even on the same day.
- Integration of microfluidics with localized AI models will significantly enhance the predictability of therapeutic efficacy, making treatments more controlled and individualized.
- ACT applications will expand beyond oncology into autoimmune diseases, offering innovative solutions for conditions such as Type 1 diabetes and benefiting a broader patient population.
In summary, microfluidic technology is poised to transform ACT therapy from “expensive and unpredictable” to “accessible and precise,” ultimately benefiting patients with cancer and autoimmune diseases.
Reference:
Wang, Zongjie, and Shana O Kelley. “Microfluidic technologies for enhancing the potency, predictability and affordability of adoptive cell therapies.” Nature biomedical engineering, 10.1038/s41551-024-01315-2. 14 Feb. 2025, doi:10.1038/s41551-024-01315-2
Related Services:
Microfluidic Chip Development Services for Cell