The brain is the most complex organ in the vertebrate body and manages the activities of our nervous system. As the upper backbone of the central nervous system (CNS), the brain can process, integrate, and coordinate received information, and then make decisions and organize the activities of various body parts. Modeling the complexity of the human brain is a great challenge due to neuronal plasticity and cellular interactions.
Traditionally, two-dimensional (2D) culture and animal models have been used for brain physiology research and drug development. Although 2D models such as Petri dishes and flasks can be used for high-throughput drug screening, they are deficient in modeling higher-order complexities such as mechanical properties of the cellular environment, and fluid conditions. Moreover, cellular behavior and drug response often are overestimated or underestimated, making it difficult to provide accurate outcome information. As far as animal models are concerned, although complex physiological models, they do not accurately reflect human physiology. One review article stated that the success rate of human anticancer drugs that have shown a good response in animal experiments is less than 8%. Additionally, sacrificing animals for research is ethically problematic and controversial.
More recently, organs-on-a-chip (OoC) has emerged as an alternative candidate for cellular experiments and drug screening. Organotypic models allow the creation of more complex cellular conditions, including self-organizing organoids and bioprinted organ cultures, allowing precise control, manipulation, and real-time analysis of cells through interconnecting channels in cells. Organ-on-a-chip enables researchers to create biologically relevant microstructures that mimic cells’ natural physiological environment, which can be used in a variety of applications, such as drug discovery, personalized medicine, or basic research on cellular mechanisms.
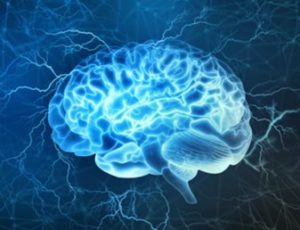
 Brain-on-a-chip (BoC) development process
Brain-on-a-chip (BoC) development process
The development direction of brain organ chips can be divided into three categories according to their high-throughput or high-content screening capabilities:
(1) A 3D high-content system that simulates a 3D brain tissue environment in terms of materials, cell types, and physiological stimuli;
(2) A interconnected multi-chip system, simulating the interaction between cells and organs;
(3) A high-throughput system that enables large-scale screening under various experimental conditions by making it compatible with traditional well-plate-based assay systems.
Brain-Organ Chip Model Features
(1) A variety of microstructures and scaffolds can be customized to accurately control the distribution of different types of brain cells;
(2) By simulating the interface interaction between blood vessels and brain tissue, the influence of mechanical factors in the body, such as fluid shear stress and mechanical compression, can be studied;
(3) Accurate control of the culture system realizes the material exchange between brain cells and between brain cells and the outside world;
(4) Cells can be stimulated using and combined chemical, magnetic, electrical, mechanical, or optical while monitoring cells in real time through pressure, flow, optical, or electrochemical sensors.
(5) The structure of the brain organ chip is close to that of the body.
Brain-Organ Chip Construction Technology
Mold lithography
- Clean the wafer surface with a cleaning solution (a mixture of sulfuric acid and hydrogen peroxide).
- Coat the wafer with photosensitive SU-8 photoresist and bake to cure.
- Use a photomask or laser to selectively expose the surface, then bake the wafer again.
- Dip the wafer into the developing solution to dissolve the SU-8 that has not been exposed to UV radiation.
- A final baking step to improve the durability of the mold.
Contact printing
- Fabricate a PDMS stamp using soft lithography.
- Immerse the stamp in a solution containing the target molecular species to allow it to be adsorbed to the surface of the PDMS stamp
- Bring the stamp into mechanical contact with the desired cell culture substrate to transfer the molecular species of interest onto the substrate.
- Repeat the process using the same or different stamps to increase the concentration of transferred molecules or to create substrates containing multiple molecular species.
Hydrogel casting
In the study of 3D cell/tissue culture and microphysiological systems, hydrogels function as scaffolds and extracellular matrix (ECM) replicas. Hydrogel casting requires a mold or master, which can be produced by photolithography, 3D printing, or machining [3]. During hydrogel casting, an aqueous polymer or prepolymer is poured into a mold and cured, typically initiated by exposure to UV light or the addition of a curing agent (curing mechanisms vary by hydrogel system). If the curing process is not too harsh, cells can be mixed into the aqueous hydrogel precursor before curing.
3D printing (Three-dimensional printing)
3D bioprinting mainly uses cells, biological hormones, growth factors, intercellular substances, and other substances to print out living tissues with biological functions. For brain-organ chips, inkjet printing or micro-extrusion printing systems are usually used. In inkjet printing, bioink is expelled in the form of droplets by microactuators. In microextrusion printing, bioinks are extruded as continuous filaments from a print head. The entire system is controlled by a machine code program, including toolpath data and tool trigger commands. In addition, 3D printers can accommodate multiple print heads in a single program, allowing the fabrication of complex systems such as structural tissues.
Applications of Brain-Organ Chip Model
Drug neurotoxicity assessment
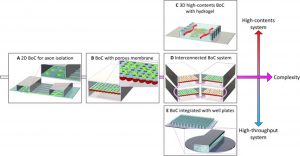
The effectiveness of central nervous system drugs is constrained by their ability to penetrate the blood-brain barrier (BBB), and the development of a reliable BBB model for drug permeability assessment can facilitate early screening of brain-targeted drugs and assess their neurological toxicity, thus accelerating drug screening process.
Brain Function Research
Brain-organ-on-a-chip models can be used to study brain functions, such as neural development and maturation. The brain-organ-on-a-chip model can be constructed through neural self-differentiation, which can simulate the migration of human neural progenitor cells induced by chemokines in the brain environment. Understanding the migration of neural progenitor cells in the complex environment of the central nervous system could drive breakthroughs in neuroregenerative therapies, brain cancer treatment, and neurodevelopmental research.
Brain Disease Model
The brain-organ chip model can also be used in the study of neurological disease models, such as neurovascular inflammation and brain tumors. By constructing cylindrical gels with hollow lumens and culturing primary human brain microvascular endothelial cells on the surface, the influence of supporting cells such as human brain pericytes and astrocytes on neuroinflammation can be studied. The dynamic three-dimensional BBB model composed of a variety of brain cells, extracellular matrix, and mechanical fluid conditions can simulate the physiological and pathological microenvironment of the brain, and provide a new way for the evaluation of the ability of clinical anti-tumor drugs to penetrate the barrier.
 Schematic diagram of various brain organ chip devices
Schematic diagram of various brain organ chip devices
Reference:
[1] Mak I W; et al. Lost in translation: animal models and clinical trials in cancer treatment[J]. Am J Trans Res, 2014, 6(2): 114.
[2] Bang S; et al. Brain-on-a-chip: A history of development and future perspective[J]. Biomicrofluidics. 2019, 13(5):
[3] Ryan J R; et al. Ventriculostomy Simulation Using Patient-Specific Ventricular Anatomy, 3D Printing, and Hydrogel Casting[J]. World Neurosurg, 2015, 84(5): 1333-1339.
[4] Lee J M; et al. Resolution and shape in bioprinting: Strategizing towards complex tissue and organ printing[J]. Appl Phys Rev, 2019, 6(1): 011307.
[5] Haring A P; Johnson B N. Chapter 6 – Brain-on-a-chip systems for modeling disease pathogenesis[J]. Organ on a Chip, 2020: 215–232.
Related Services:
Brain-On-A-Chip Model Development Service
CBLMF™ 3D Tissue and Organ-On-A-Chip Model Development Services