Background
PCR is widely regarded as the gold standard for nucleic acid testing (NAAT) due to its excellent sensitivity, tolerance to nucleotide variations, and ability to perform multiplex detection. The development of ultrafast PCR systems requires the use of sophisticated instrumentation. To facilitate rapid PCR for point-of-care testing (POCT) applications, microfluidic technology has been extensively studied for its advantages in reagent distribution, reaction volume reduction, and automation. Among these, digital microfluidics (DMF) based on dielectric wetting (Electrowetting-on-dielectric, EWOD) enables precise control of single droplets, and its high configurability holds promise for developing efficient workflows in lab-on-a-chip applications. However, EWOD-based digital microfluidic systems are prone to bubble formation at high temperatures during PCR thermal cycling. In addition, rapid and frequent movement of droplets between thermal zones can lead to dielectric breakdown, and inaccuracies temperature sensing of droplets by temperature sensors in ultrafast thermal cycling reactions may damage the EWOD PCR durability. These technical challenges hinder the application of EWOD for on-site diagnosis of rapid PCR.
Recently, the journal reported an ultra-fast PCR based on a dielectric wetting digital microfluidic platform, which integrates techniques such as dual-layer heating, porous superhydrophobic membrane, temperature isolation tank, soluble chemical temperature sensor, and other technologies to comprehensively solve the problem of dielectric wetting. These solutions comprehensively address the stability issues in PCR thermal cycling, allowing PCR to complete 40 thermal cycles within 3.7–5 minutes and achieving sensitivity comparable to traditional PCR. The relevant results were published under the title “Sub-5-Minute Ultrafast PCR Using Digital Microfluidics” in the international academic journal Biosensors and Bioelectronics (DOI: 10.1016/j.bios.2023.115711).

Main Content of the Research
In this article, the research team developed an integrated EWOD digital microfluidic PCR system that solves the stability problem of fast PCR based on the EWOD platform. The system principle and structure diagram are shown in Figure 1(a). The EWOD PCR system uses dielectric wetting-mediated droplet movement between different temperature zones to complete the PCR thermal cycle.
Since the droplets complete the PCR thermal cycle while constantly moving rapidly, conventional temperature sensors are not suitable for detecting the temperature in the moving droplets. In addition, the temperature deviation between the sensor and the actual temperature of the droplets can easily lead to a reduction in on-chip PCR efficiency. Therefore, the research team used a soluble temperature sensor (sulforhodamine B) to perform precise temperature calibration and regulation within droplets to achieve efficient on-chip EWOD PCR. The high-temperature denaturation temperature zone uses top and bottom double-layer heating to reduce the vertical temperature difference of droplets and ensure the stability and accuracy of droplet PCR.
To effectively eliminate dielectric breakdown and bubble generation caused by high temperatures, the research team used polydimethylsiloxane (PDMS) as a dielectric layer to protect the electrode from breakdown at high temperatures. Porous expanded PTFE (ePTFE) is covered with semi-cured PDMS as a hydrophobic layer. Due to its porous and expandable properties, it is not easily broken due to thermal expansion at high temperatures, thus eliminating the damage caused by the surface hydrophobic layer.
To speed up the reaction speed of droplet PCR, a thermal isolation groove (TIG) is set up around the top high-temperature film heater (see Figure 1(b)), so that the two temperature zones of denaturation and annealing/extension are at the same level. The temperature difference increases, significantly reducing the shuttle movement distance of PCR droplets between the two zones. Finally, a new type of moving electrode interlocking configuration reduces the limitation of the moving electrode on the droplet size, allowing droplets larger than 0.2 µL to move continuously at a temperature difference of greater than 30 °C without being affected by the thermal capillary. The influence of this effect hinders the movement of droplets to high-temperature areas.
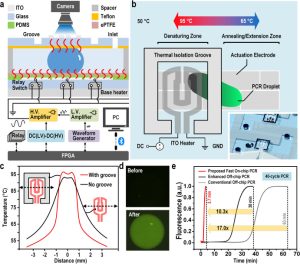 Figure 1. Schematic Diagram of An Ultra-Fast PCR System Based on Dielectric-Wetting Digital Microfluidics
Figure 1. Schematic Diagram of An Ultra-Fast PCR System Based on Dielectric-Wetting Digital Microfluidics
The above technology ensures that the PCR droplets move stably in different temperature zones with accurate temperature control for thermal cycling. The integrated EWOD digital microfluidic system developed by Hede achieved 40 cycles of ultra-fast PCR in 3.7 minutes (Figure 2 (a)). The use of a reversible hot-start reagent (ThermaStopTM) eliminates non-specific amplification of on-chip PCR. Serial dilutions experiments verified that the sensitivity of EWOD ultra-fast PCR was consistent with off-chip traditional PCR, and the PCR efficiency reached 95.31% (Figure 2 (b-c)). The ultrafast PCR digital microfluidic system platform proposed in this study provides practical application opportunities for nucleic acid detection, drug resistance gene screening, and melting curve analysis of different infectious pathogens.
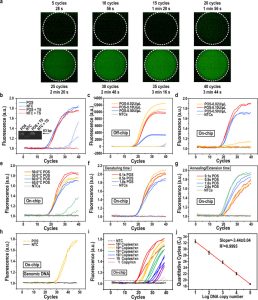 Figure 2. On-Chip PCR Based on Digital Microfluidics
Figure 2. On-Chip PCR Based on Digital Microfluidics
Summary
This study introduces a robust ultrafast PCR system based on EWOD digital microfluidics. The combination of new materials and technologies used in this study effectively enhances the durability of the EWOD chip and can achieve at least 240 droplet shuttles on a temperature gradient exceeding 30°C. Ultrafast EWOD PCR in a volume of 0.6 μL achieves 40 thermal cycles in 3.7–5.0 min. It is 10–17 times faster than the traditional PCR method. This EWOD system also provides the possibility for NAAT to be used for the detection of infectious pathogens, drug resistance molecular detection, forensics, and other PCR-based practical applications.
Reference:
Wan L, Li M, Law MK, Mak PI, Martins RP, Jia Y. Sub-5-Minute Ultrafast PCR using Digital Microfluidics. Biosens Bioelectron. 2023;242:115711. doi:10.1016/j.bios.2023.115711
Related Services: