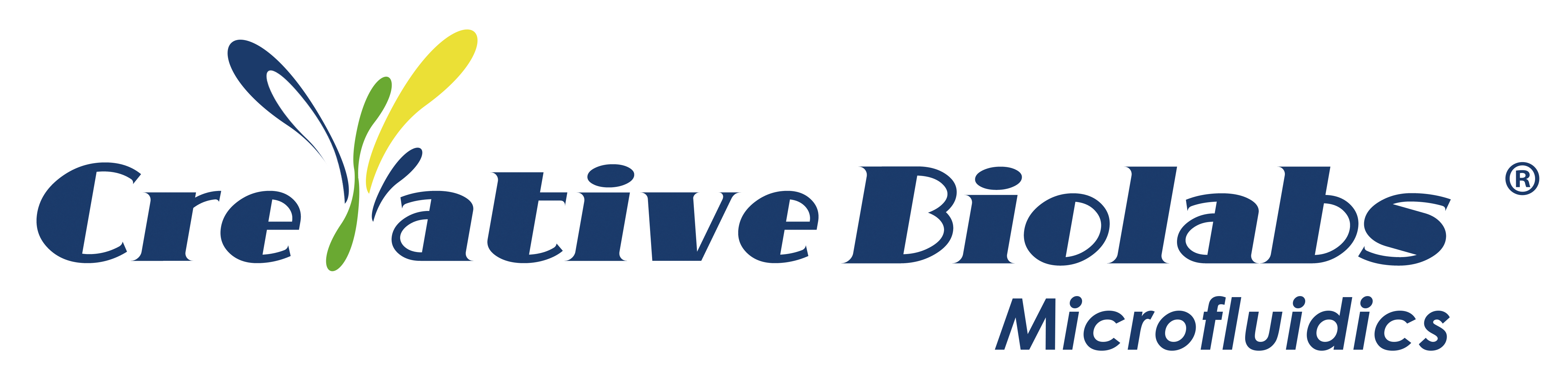As the main organ of the human respiratory system, the main function of the lungs is to transport oxygen from the air into the blood and release carbon dioxide. The lungs are mainly composed of the trachea, bronchi, bronchioles, alveolar ducts, and alveoli. The risk of respiratory disease is on the rise globally, with nearly 4 million deaths from chronic respiratory diseases each year. In addition, the incidence of potential pandemic viruses such as influenza A virus, MERS-CoV, severe acute respiratory syndrome coronavirus (SARS-CoV), and now novel coronavirus (SARS-CoV-2) continues to increase. Therefore, there is an urgent need to develop new preclinical approaches to accelerate the development of effective therapeutic and preventive drugs. This requires good physiologically relevant respiratory disease models and drug testing models.
Methods for conventional physiology are two-dimensional (2D) monolayer cell culture or transwell-based immersion culture [1], but these models cannot accurately replicate in vivo three-dimensional (3D) cell structure, cell-cell interactions, air exposure environment of alveolar cells, and physiological functions at the organ level. The lung-on-a-chip (LOC) model that has emerged in recent years combines microfabrication technology with modern tissue engineering, and has become a new in vitro drug screening tool by simulating the complex mechanical and physiological behavior of the human lung.
 Characterization of Human Respiratory Chip and Its Infection to Influenza Virus
Characterization of Human Respiratory Chip and Its Infection to Influenza Virus
Features of the lung-on-a-chip model
Using Primary Human Cell Models
Co-culture of primary human epithelial and endothelial lung cells allows for cell-cell interactions and overcomes translational challenges caused by species differences in animal models.
Provides mechanical force associated with tissue
The dynamic microenvironment includes media flow, and changing the structure of the membrane under vacuum to mimic the expansion/contraction of alveoli during respiration. Modeling on a chip can improve function and form.
Reproduce the gas-liquid interface
The lower gel network layer and the upper gas phase pipeline network layer are used to form the cell gas-liquid growth interface, simulating the actual state of human lung cells exposed to the gas.
Build in vitro disease models
A series of key pathophysiological processes such as lung barrier dysfunction, immune cell adhesion, inflammatory factor release, and lung endothelial cell injury caused by diseases can be simulated in vitro, providing a new research platform for exploring respiratory diseases.
Lung chip construction technology
Mold manufacturing and surface treatment
First, use the design software to design the required mold in CAD, and then use the 3D printer to manufacture the mold. The 3D-printed molds were cleaned with isopropanol and then dried with high-pressure air after printing. Afterward, the mold requires a 3 min post-cure. Soak the prepared mold in 100% ethanol for 2 hours to ensure that uncured monomers and oligomers on the surface of the mold are removed. Then, oxygen plasma treatment was performed for 2 min. Finally, the surface of the mold was vacuum-dried with trichlorosilane for 1.5 h to complete silanization.
Equipment and device manufacturing
After surface-treating the resin mold, prepare a mixture of PDMS base and curing agent (10:1 ratio). The mixture was degassed in a desiccator for 20 min and poured into treated molds. Cure the PDMS in a hot air oven at 45 °C for 4-5 h. Next, gently remove the PDMS layer from the mold, trim it to the desired shape, and punch holes for the inlet and outlet. Thoroughly clean the PDMS layer at least 3 times with isopropanol and ethanol, air drying between each wash. The polycarbonate membrane was cut into a square large enough to cover the central circular hole and carefully placed under the PDMS layer. The contact surfaces of the upper and lower PDMS layers are plasma-treated for 1 min and precisely aligned to ensure a perfect bond. The bonded chips were then placed in the oven again at 45 °C for 4 hours to ensure bonding. Finally, test for leaks using food dye.
Cell culture
The human airway epithelial cell line Calu-3 was cultured in DMEM/F12 mixed medium supplemented with 10% fetal bovine serum (FBS), 1% l-glutamine, 1% penicillin, and 1% non-essential amino acids. Sterilize the chip by rinsing the channels with 70% ethanol, then dry it in an oven with hot air. Expose it to UV light in a biosafety cabinet for 30 min before extracellular matrix (ECM) coating. After coating the membrane with ECM, the chip was placed in a humidified incubator at 37°C and 5% CO2 for 2 h before seeding cells. Remove residual ECM coating in the channel by flowing through a fresh medium. After seeding the cells, keep the chip at 37 °C and 5% CO2. Change the medium in the upper and lower channels daily until the cells are confluent (approximately day 5). Once confluence is reached, media is aspirated from the upper channel to allow cells to grow at the air-liquid interface. Then connect the lower channel to a syringe pump with a flow rate of 30 µl/h to keep the fresh medium flowing.
Applications of the lung-on-a-chip model
Physiological Study of the Lung
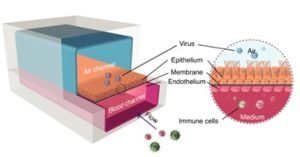
The lung-on-a-chip can replicate the functional units of the human lung and reconstruct the three-dimensional microstructure, dynamic mechanical activity, and comprehensive physiological functions of the alveolar-capillary interface. The lung-on-a-chip pictured below illustrates that the introduction of air in the epithelial compartment enables cells to remain viable for extended periods of time. In addition, this approach also increases the electrical resistance of different tissue layers, improving structural integrity and normal barrier permeability compared to cells cultured in liquid media.
 On-chip formation and mechanical stretching of the alveolar-capillary interface
On-chip formation and mechanical stretching of the alveolar-capillary interface
Toxicology research
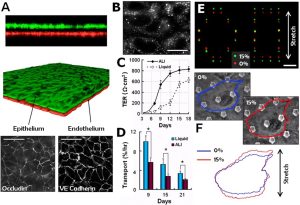
The application of organ-on-a-chip to the study of toxicological salts has the advantages of high throughput, high sensitivity, and good repeatability. Some studies have used the lung chip as the research object, and found that nanoparticle exposure and physiological breathing motion can induce and accelerate the toxic effect on the lung, indicating the importance of mechanical motion on lung function. These results are similar to those found in an in vivo lung ventilation-perfusion mouse model. The proliferation of lung-on-a-chip applications promises to reduce or even replace the need for animal research.
Disease model
The lung-on-a-chip model also has great potential in precision medicine research and the treatment of cancer. It can be used for a variety of purposes, such as oncogene modeling, screening chemotherapy drugs in a personalized manner, and studying the chemosensitivity of drug candidates. For example, non-small cell lung cancer cell lines and lung fibroblasts can be used to create co-culture models for high-throughput drug screening. In addition, chip models for simulating lung inflammation, pulmonary edema, and pulmonary infectious diseases have been gradually established and perfected, which is of great significance for the study of the pathogenic mechanism of lung diseases and the development of new drugs.
 Lung-on-a-chip disease model
Lung-on-a-chip disease model
Reference:
[1] Huh D; et al. From 3D cell culture to organs-on-chips Trends[J]. Cell Biol, 2011, 21(12): 745-754.
[2] Si L; et al. A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics[J]. Nat Biomed Eng, 2021.
[3] Huh D; et al. Reconstituting organ-level lung functions on a chip[J]. Science, 2010, 328: 1662–1668.
[4] Huh D D. A human breathing lung-on-a-chip[J]. Ann Am Thorac Soc, 2015;12: S42–S46.
[5] Constant S; et al. Advanced human in vitro models for the discovery and development of lung cancer therapies, drug discovery and development. 2015. DOI: 10.5772/60606.
[6] Jesus S; et al. Lung-on-a-chip: the future of respiratory disease models and pharmacological studies[J]. Crit Rev Biotechnol, 2020,1-18.
Related Services: