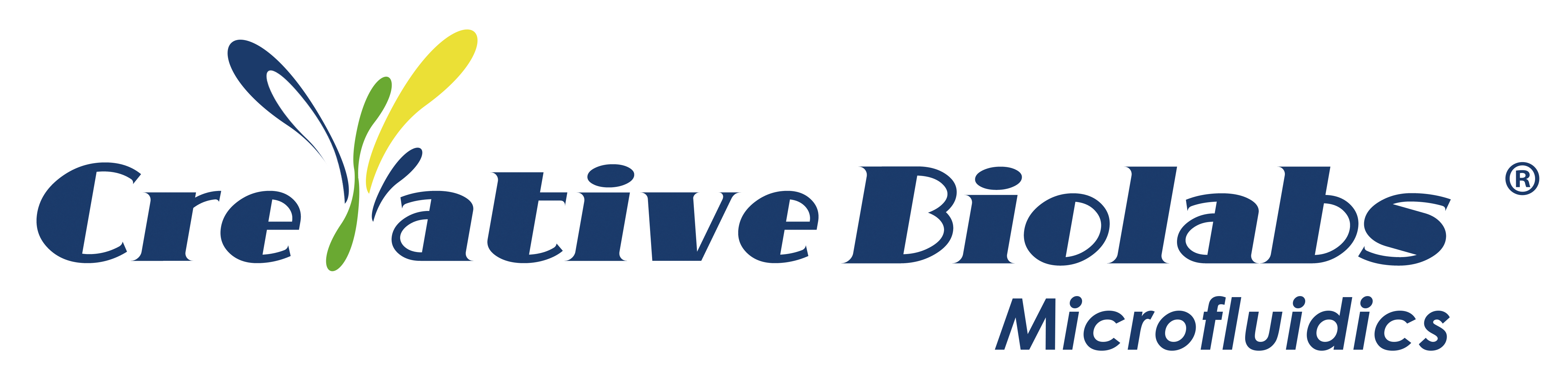The kidney is an important excretory organ of the human body. It is responsible for removing metabolites and certain wastes and poisons from the body. At the same time, it retains water and other useful substances through the reabsorption function, such as protein, amino acid, glucose, and sodium ions, to regulate water and electrolyte balance and maintain acid-base balance. The kidney is also an endocrine organ that can secrete various hormones and biologically active substances such as renin, erythropoietin, and prostaglandins. Therefore, research on renal physiology and disease-related topics is very extensive.
Major kidney diseases
- Glomerulonephritis (GN)
It is an inflammatory disease that damages the glomeruli. One of the main features is hyperfiltration and renal impairment. GN primarily results in structural defects in podocytes, one of the cell types that ensure normal renal function.
- Polycystic Kidney Disease (PKD)
It is a group of monogenic disorders that lead to the development of renal cysts. The cysts destroy the renal tubular epithelium, leading to fibrosis, renal structural disturbances, and ultimately renal failure. To date, the exact mechanism leading to cyst formation has not been fully elucidated.
- Renal fibrosis
It is a direct consequence of the limited regenerative capacity of the kidney after injury. Fibrosis involves excessive accumulation of extracellular matrix (ECM), often resulting in loss of function as normal tissue is replaced by scar tissue.
- Kidney stones
It is a common urinary problem affecting approximately 10% of men and 6% of women and is increasing in prevalence in many developed countries. The factors underlying kidney stone formation remain unclear. Therefore, delving deeper into this complex mechanism may pave the way for the development of new strategies to prevent the disease.
Kidney Disease Research Model
Nephrotoxicity is one of the main causes of drug failure and drug development failure. Only about 2% of drug development failures can be screened out in the preclinical stage, and more than 20% of new drugs have serious adverse reactions only after the clinical stage. The reason why preclinical studies cannot predict nephrotoxicity well is the limitations of current preclinical cell culture and animal models. Cell culture methods simply grow living cells on Petri dishes, which lack blood flow and have different conditions from cells in a real in vivo environment. Animal models though allow for studies to be performed in a complete physiological environment, tubular secretion information obtained from animal studies cannot always predict human responses due to species differences. In contrast, the organ-on-a-chip, which can truly simulate human organs, has become an excellent solution. It has minimal functional units, uses primary human cells, and can even simulate real human organs’ 3D structure and flow conditions.
 Schematic diagram of a human kidney organ-on-a-chip
Schematic diagram of a human kidney organ-on-a-chip
Kidney Organ Chip Construction Technology
- Fabrication of Microfluidic Chips
1. Mask design
Use software to design mask patterns, and then use film to make the designed patterns into masks.
2. Template making
(1) Cleaning the glass substrate
30% hydrogen peroxide, ammonia water, and deionized water were prepared in a ratio of 1:1:5 to make an alkaline washing solution, and a 75 mm x 75 mm optical glass was placed in the alkaline washing solution, and heated in a water bath at 75 °C. After taking it out, ultrasonically clean it for 5 minutes, replace it with deionized water and repeat the cleaning three times, and then take out the optical glass to air dry.
(2) Uniform glue
After the photoresist was ultrasonically removed at 50 °C for 1 h to remove air bubbles, it was left to stand at room temperature for 30 min, and then the photoresist was loaded into the center of the optical glass with a plastic dropper, and the glue was evened out with a homogenizer.
(3) Pre-bake
After uniform coating, the optical glass substrate was baked at 95 °C for 30 min, and after cooling down to room temperature, the hardness of the photoresist was detected and the thickness of the photoresist was measured.
(4) Exposure
Cover the photoresist side of the optical glass with a mask, and use a UV exposure machine to crosslink the photoresist. The required exposure energy is related to the exposure time and the thickness of the photoresist.
(5) Post-baking
Place the exposed optical glass substrate on a horizontal hot plate and bake at 95 °C. After the mask pattern is revealed, place the optical glass substrate at room temperature to cool down.
(6) Development
Put the cooled optical glass substrate in ethyl lactate to remove the uncrosslinked photoresist, and check the development degree of the template with isopropanol, if there is white precipitation, continue to wash the template in ethyl lactate, and repeat the process until no white precipitate appears after placing the template in isopropanol.
(7) Hard film
After drying the isopropanol on the surface of the template, check the details of the template with a microscope, then place it in an oven at 120 °C for 2 h to harden the film, cool down slowly, and cool the template to room temperature.
3. PDMS chip processing
The PDMS prepolymer and PDMS curing agent were mixed at room temperature for 30 min at a volume ratio of 10:1, and then the mixed PDMS colloid was poured on the template, and the air bubbles in the PDMS colloid were removed by vacuum. Subsequently, the poured PDMS was placed in an oven and baked at 80 °C for 1 h to solidify. The cured PDMS is peeled off from the template, punched with a puncher, and then the PDMS chip is sealed according to the design requirements using a plasma cleaner.
- Kidney tubule chip assembly
Monolayer renal tubular epithelial cell chip assembly
According to the needs of the experiment, the porous membrane planted with renal tubular epithelial cells was sandwiched between the blood pole piece and the urine pole piece, and fixed with screws and nuts. “Artificial urine” flows on the side of renal tubular epithelial cells, and “artificial blood” flows on the other side, and the two fluids exchange substances at the porous membrane with cells.
Kidney tubule peritubular chip assembly
The porous membrane planted with renal tubular perivascular endothelial cells and renal tubular epithelial cells is sandwiched between the blood pole block and the urine pole block in a back-to-back manner, and fixed with screws and nuts. Artificial urine flows at a flow rate of 1 L/min on the side of renal tubular epithelial cells, and artificial blood flows at a flow rate of 1 L/min on the side of renal tubular vascular endothelial cells. The two flows occur at the porous membrane with cell material exchange.
Application of Kidney Organ Chip Model
Drug evaluation
About 20% of episodes of acquired renal failure are drug-induced. The kidney-on-a-chip model can reflect the dynamic changes of drugs in the body and the real response of human organs to drugs, and better make up for the shortcomings of existing in vitro models or animal experiments that deviate from the human body.
Disease research
Kidney-on-a-chip offers new opportunities to study kidney physiology and kidney disease as well as nephrotoxicity. For example, the main pathological changes of glomerular damage in the early stage of diabetic nephropathy can be reflected by constructing a kidney chip model containing primary glomerular tissue, matrix components, and vascular-like mechanical fluid.
Toxicological evaluation
Due to the complexity of the human body, the existing animal experiments and in vitro evaluation models cannot accurately reflect the human body’s response to various adverse factors. The kidney-on-a-chip model can better simulate the real response of the human body to pharmaceutical compounds and toxins, and significantly reduce the cost and time of toxicity assessment, so it is a very promising alternative strategy for animal experiments and in vitro evaluation models.
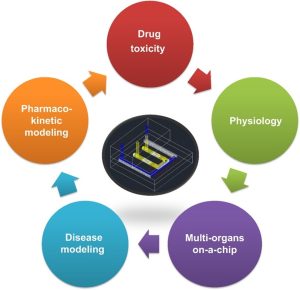 Kidney-on-a-Chip Applications
Kidney-on-a-Chip Applications
Reference:
[1] H Laverty, et al. How can we improve our understanding of cardiovascular safety liabilities to develop safer medicines?[J]. Br J Pharmacol, 2011, 163(4): 675-693.
[2] Scales C D, et al. Prevalence of Kidney Stones in the United States[J]. Urol,2012, 62: 160–165.
[3] Paoli R, Samitier J. Mimicking the Kidney: A Key Role in Organ-on-Chip Development[J]. Micromachines. 2016, 7(7): 126.
[4] Naughton C A. Drug-induced nephrotoxicity[J]. Am Fam Physician,2008, 78: 743–750.
[5] Kim S, Takayama S. Organ-on-a-chip and the kidney[J]. Kidney Res Clin Pract, 2015, 34(3): 165-169.
Related Services: