Drug discovery and development is a long-term, high-investment, and high-risk process. In the preclinical stage, toxicity issues of drug candidates are often the main reason for development failure. Therefore, it is very important to identify drug toxicity and adverse drug interactions in the early stages of drug development. Comprehensive and in-depth toxicology research can not only play an early warning role in the early development of drugs, but also effectively reduce the potential risk of market withdrawal after listing.
Common drug toxicity manifestations:
- Digestive system reaction: It is also the most common drug toxicity reaction, usually manifested as nausea, vomiting, abdominal pain, diarrhea, etc., and can cause blood in the stool, peptic ulcer, or even death in severe cases.
- Liver damage: Elevated transaminases, jaundice, hepatomegaly, and pain in the liver area are common, and severe cases include hepatitis, liver necrosis, and even death.
- Kidney damage: proteinuria, hematuria, renal insufficiency, etc. are common, and severe cases could be nephritis, renal failure, and death.
- Nervous system reactions: Insomnia, headache, dizziness, agitation, hallucinations, nystagmus, vision loss, hearing loss, etc. are common, and severe cases may have convulsions, permanent deafness, and mental confusion.
- Blood system reaction: Anemia and leukopenia are common, and in severe cases, drug toxicity can cause aplastic anemia, leukopenia, and immune function decline that can cause uncontrollable secondary infections.
- Cardiovascular system reaction: Arrhythmia, myocardial damage, abnormal blood pressure, and cardiac function inhibition are common.
In preclinical research, the content of safety research mainly includes:
- Acute toxicity test
- Subacute and subchronic toxicity tests.
- Chronic toxicity test
- Genetic toxicity research.
- Carcinogenicity studies.
- Studies on reproductive toxicity (such as teratogenicity and mutagenicity)
In Vitro Biological Testing Technology for Drug Toxicity
For drug toxicity testing, traditional animal experiments have many limitations. Not only are they expensive and time-consuming, but they also have species differences from humans and cannot always identify toxic effects relevant to humans. In vitro biological testing of drug toxicity can strictly control the experimental conditions. Compared with animal experiments, these systems can detect drug toxicity at the cell, tissue, and organ levels in high throughput, and better predict the response of drugs to humans.
Cell-Based Phenotype Analysis
High-throughput assays use cultured cells to measure some of the gross phenotypic outputs relevant to predicting toxicity, such as T lymphocyte proliferative responses, based on the ability of T cells derived from peripheral blood or spleen to generate blastic activation in response to specific antigens proliferation. Cytotoxicity assays can also be assessed based on changes in cell membrane permeability, and the following methods are commonly used:
- MTT method: MTT is a tetrazolium salt dye. Under the action of succinate dehydrogenase (SDH) and cytochrome C, the tetrazolium ring will crack and form purple formazan crystals. The amount of produced formazolium crystals is directly proportional to the number of viable cells, and the cell viability can be evaluated by spectrophotometer after dissolving formazan in DMSO.
 Cell metabolism produces purple formazan
Cell metabolism produces purple formazan
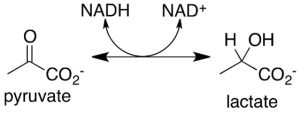
- LDH method: Lactate dehydrogenase (LDH) is a stable protein that widely exists in the cytoplasm of normal cells. Once the cell membrane is damaged, LDH is released outside the cell; LDH catalyzes lactate to form pyruvate and can react with INT (tetrazolium salts) to form a purple crystalline substance, which can be detected by a 500nm microplate reader. Therefore, by detecting the activity of LDH in the cell culture supernatant, cytotoxicity can be detected.
Gene expression and protein secretion assays
Specific toxic mechanisms are determined by measuring the expression of specific genes or the secretion of specific proteins, which are considered biomarkers of specific toxic mechanisms in humans.
- Gene expression readouts can use engineered reporters to drive the expression of easily detectable reporter genes such as luciferase through artificial promoters in response to the activation of toxic pathways or endogenous gene circuits. Engineered reporter genes allow for easy standardized and reliable readouts, and multiple biological pathways can be detected using such assays.
- Protein secretion assays are of particular value for measuring the activity of immunotoxins that induce the expression and secretion of specific inflammatory cytokines (such as TNF-α, IL1, and IL6) in leukocytes. Cytokines are usually measured by immunoassay.
Organ model testing
Cell-based assays have many limitations. Monolayers of cells grown in culture dishes, and in vitro environments can change their phenotype within hours, inevitably making them poor models for human tissue. As a result, co-culture methods of multiple cell types, or the culture of whole organs as slices or cell aggregates, have been developed, collectively referred to as organotypic models. For example, the human tissue organ on a chip (OOC) produced in combination with microfluidic technology can reproduce the internal organ environment in vitro, which can not only effectively reflect the physiological effects of drug exposure, but also allows precise control just like in vitro experiments. Creative Biolabs has a variety of biological platforms for in vitro organ models, including but not limited to skin organ models, eye organ models, lung epithelial organ models, liver organ models, and nerve organ models. These models are of great value in predicting drug toxicity.

Related Services: