Cardiovascular diseases (CVD), a group of diseases affecting the heart and vascular system, are among the most common causes of death worldwide. In Europe alone, cardiovascular disease kills more than 4 million people every year. The most common underlying pathology of cardiovascular disease is atherosclerosis, which leads to cardiac and peripheral arterial ischemia. The disease process involves the interaction of multiple cell subtypes, including endothelial cell dysfunction, leukocyte and macrophage-mediated inflammatory responses, and smooth muscle cell dedifferentiation or apoptosis. Over the years, a variety of drugs have been developed for diseases of the heart and vascular system, but the number of patients affected continues to increase, and the development of new and improved treatments is urgently needed.
In recent years, organs-on-chips (OoCs) have gradually become a powerful new tool to fill the translation gap from animal models to human diseases. OoC relies on microfluidic technology, which has the potential to achieve physiological similarity in vitro. Microfluidic technology allows the fabrication of systems composed of human cells that recapitulate some aspects of organ function and can be used to probe key molecular or cellular events in physiologically relevant structures to explore mechanisms of acute or chronic disease development.
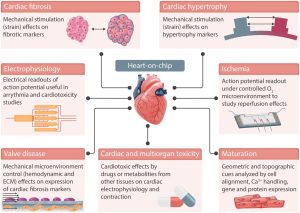 Different applications of the heart-on-a-chip
Different applications of the heart-on-a-chip
Heart-on-a-chip models currently focus on establishing biomimetic functions of the heart, particularly the co-culture of multiple cell types, for example, cardiomyocytes and cardiac fibroblasts, and electromechanical stimulation of cells cultured on these systems. Heart-on-a-chip models aim to recapitulate the complex microenvironment that cells experience in the heart, and growing 3D cell cultures in these devices allows for increased biological complexity. Control over the microenvironment and cultured cell type allows for the recapitulation of relevant aspects of specific diseases.
Characteristics of Cardiovascular Organ Chip Models
Real-time dynamic cell culture and result analysis.
Low cell volume and low reagent consumption.
Flexible customization and high integration with other devices.
Ability to simulate the systolic motion of the heart (with controllable shear stress and mechanical stress provided).
Cardiovascular Organ Chip Construction Technology
1. Chip Fabrication
(1) A chip holder model with microwells was first generated in AutoCAD and then fabricated into a single SU-8 master using standard soft lithography.
(2) Mix PDMS and cross-linking agent at a ratio of 1:15, mold on SU-8 master and let it cure at room temperature for 2-3 days.
(3) The patterned PDMS molds for the base layer and upper layer of the 3D scaffold were covered on glass slides and flat PDMS sheets, respectively.
(4) The POMaC solution was injected into the patterned network through the inlet and outlet and left overnight at room temperature. The POMaC solution fills the entire PDMS mold, including the vertical columns extending from the main grid network. Since PDMS is porous and allows air to escape, apply gentle positive pressure at the inlet to push trapped air out of the mold.
(5) Next, the injected POMaC solution was cross-linked with 60% (w/w) PEGDM/POMaC mixed solution under UV light for 4 min, or 10 min if PEGDM was not added. Afterward, the PDMS mold is opened and the patterned polymer structure is exposed.
(6) Repeat the previous steps to bond additional patterned POMaC flakes to the established base structure.
(7) Finally, the fabricated scaffolds were immersed in phosphate-buffered saline (PBS) to release them from the slides and incubated overnight at room temperature to leach out the PEGDM porogen, patterning multiple in parallel on a single slide.
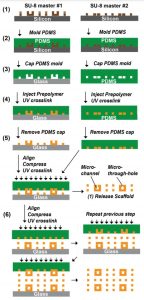 Cardiovascular Organ Chip Manufacturing Process
Cardiovascular Organ Chip Manufacturing Process
2. Endothelialization and Tissue Assembly
(1) The scaffold was coated with 0.2% (w/v) gelatin in PBS for 2 h before assembly to enhance the attachment of cells on the vessel-on-a-chip scaffold and in the internal network.
(2) Perfuse 10–20 µl of concentrated endothelial cell suspension (approximately 25 million cells/ml) into the built-in network of the scaffold for 1 min, stop the flow and let the cells attach under static conditions for 2 h.
(3) Then add 1 ml of endothelial cell medium to the inlet well to flush out unattached cells, thus perfusing at a flow rate of less than 0.7 µl/min to exert minimal pressure on the cells while providing sufficient medium to the cells.
(4) After 2 h of incubation, 3 ml of EC medium was added, and the flow rate was increased to a day-average perfusion rate close to 0.7 µl/min. Endothelial cells proliferated overnight and formed a confluent network.
(5) To create human cardiac tissue chips, hESC-derived cardiomyocytes were seeded with 15 µl Matrigel at a concentration of 1–200 million cells/ml onto each chip holder.
(6) After gelation at 37°C for 30 min, add 1 ml of cardiomyocyte culture medium to the middle well. After seeding parenchymal cells, add an additional 4 ml of endothelial cell culture medium to the inlet wells to increase the medium perfusion rate back to a one-day average perfusion rate of 0.7 µl/min.
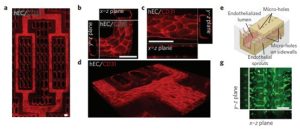 Schematic diagram of a cardiovascular chip
Schematic diagram of a cardiovascular chip
Applications of Cardiovascular Organ Chip Model
Drug evaluation
In the process of drug evaluation, it is necessary to understand the impact of drugs on the biological function of the heart. When constructing the heart organ model, the stress sensor is integrated to obtain the contraction force generated by the beating heart cells in real time. This 3D heart model with real-time monitoring function has important application prospects in the evaluation of myocardial toxicity. In addition, using the 3D cardiac microarray of cardiomyocytes to construct a chip can realize the mechanical and electrophysiological characterization of the heart, reflecting the dynamic changes of the drug in the cardiovascular system and the true response of the heart organ to drug stimulation.
Cardiac Function Research
Cardiac development and physiological function depend on the response of cardiac cells to mechanical stress from the blood. These stimuli can induce changes in cellular architecture, contractile function, and gene expression. For example, embryonic fibroblasts are cultured on a flexible membrane, in which fluid shear stress is supplied to the system through a pump, and mechanical stress is generated through valve control to cause the membrane stretch to simulate cardiac load and study cardiac function.
Cardiovascular Disease Research
In the field of disease research, organ chips can reproduce the characteristics of human diseases and study the pathological mechanisms of diseases involving multiple factors. Cardiovascular organ-on-a-chip models can be used in the study of heart diseases, such as myocardial infarction (MI), hypertension, heart failure, and atherosclerosis. A cardiac organ-on-a-chip model can be constructed using muscle membranes. The development of a similar single-chip model of heart failure can help to further understand the mechanism of heart disease and provide valuable research objects for the pharmaceutical industry.
Reference:
[1] Townsend N; et al. Cardiovascular disease in Europe: epidemiological update 2016[J]. Eur Heart J, 2016, 37: 3232-3245.
[2] Basatemur G L; et al. Vascular smooth muscle cells in atherosclerosis[J]. Nat Rev Cardiol, 2019, 16: 727-744.
[3] Paloschi V; et al. Organ-on-a-chip technology: a novel approach to investigate cardiovascular diseases[J]. Cardiovasc Res, 2021, cvab088.
[4] Zhang B Y; et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis[J]. Nat Mater, 2016, 15: 669-678.
Related Services:
Microfluidic Chip Development Services for Organ-On-A-Chip
Cell Culture and Organ-On-A-Chip Model Development Services