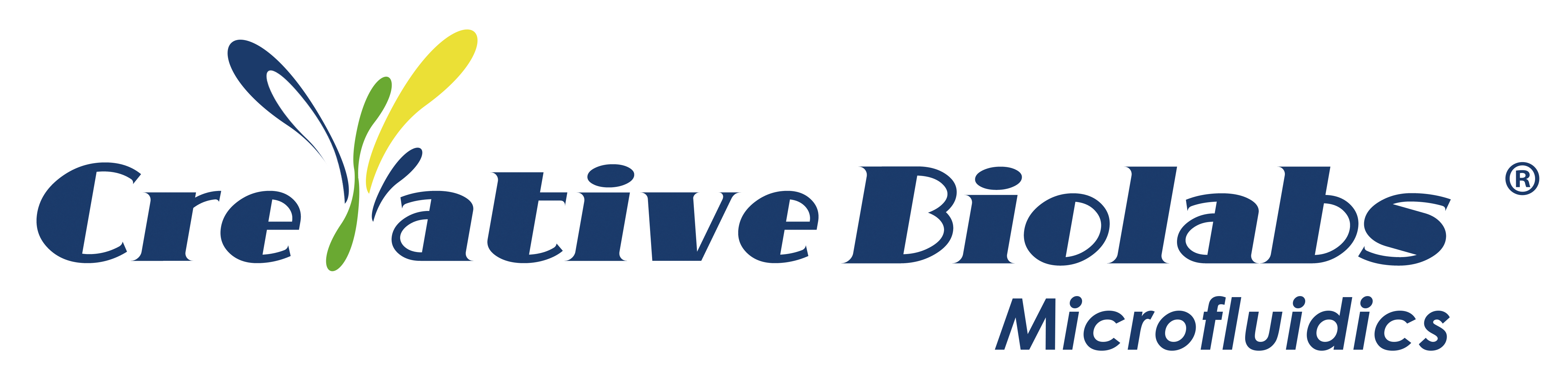As a new type of cell experiment tool, the organ chip integrates chip technology and chemical analysis technology, and forms a 3D organ model system in vitro based on different principles such as cell self-assembly and engineering design, which can partially overcome the limitations of traditional animal models. It has gradually become an important drug evaluation model.
 Organ-on-a-chip Applications
Organ-on-a-chip Applications
As a professional agent providing organ chip research and development services, Creative Biolabs provides various service types for organ chip development, testing, and evaluation. Taking advantage of advanced technology platforms and a professional research team, the company has established an organ chip detection service system involving various fields such as medical evaluation and transport function determination.
Detection Technology Based on Organ Chips
Secretion/effluent assay
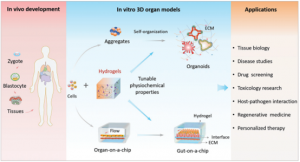
Organ chip-based secretions/effluents include peptides, proteins, amino acids, miRNA, DNA, RNA, etc. Compositional analysis of these microparticles allows for rational inferences about cell growth, testing response to drugs, or high-throughput drug screening, among others. In addition, the measurement of secretions can also provide potential treatment methods for diseases. For example, for some diseases that are not easy to be directly administered to target organs, the secretion of hormones in other organs can be adjusted to indirectly treat diseased organs. Measurement of secretions/effluents by an organ chip-mass spectrometry platform allows cell secretions to be recovered during cell culture for component composition analysis. This technique enables kinetic studies over a sustained period of time to determine cell viability and function. The introduction of high-resolution mass spectrometry provides a powerful means for the qualitative and quantitative detection of signaling factors in cell signaling. It can also test the direct impact of drugs on the human microbiome before medication, which is helpful for the development of personalized medicine.
 Microfluidic perfusion system for insulin secretion fingerprint analysis
Microfluidic perfusion system for insulin secretion fingerprint analysis
Functional Transport Studies
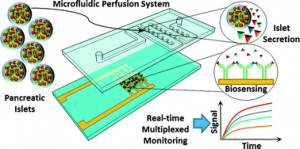
Creative Biolabs can provide a variety of drug transport function studies to evaluate the effectiveness and safety of new drugs. Currently, a series of transport function tests for the liver, intestine, and peritoneum are provided. Take Caco-2 cell-based intestinal transit research as an example, Caco-2 cells were first cultured using chip membranes, which showed a low permeability coefficient when cyclophosphamide was absorbed by the intestinal wall. Therefore, by comparing the permeation parameters, the translocation can be accurately judged. In addition, the joint use of multiple analytical instruments can further ensure the reliability and comprehensiveness of the data, such as high-performance liquid chromatography (HPLC), real-time fluorescent quantitative nucleic acid amplification detection system (qPCR), and enzyme-linked immunosorbent adsorption (ELISA).
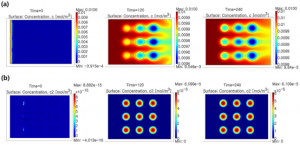 Mathematical Simulation of Metabolic Reactions and Transport Phenomena in Liver-on-a-Chip
Mathematical Simulation of Metabolic Reactions and Transport Phenomena in Liver-on-a-Chip
Determination of differentiation status
Organ chips can simulate the morphology, proliferation, differentiation, and migration of cells in vivo, and can conduct research on different disease model states in a similar tissue microenvironment. For example, human embryonic stem cells (ES) and induced pluripotent stem cells (iPS) can be induced to differentiate to adapt to specific needs for organ-on-a-chip development. The differentiation state of cells can be characterized by the activity of alkaline phosphatase (ALP).
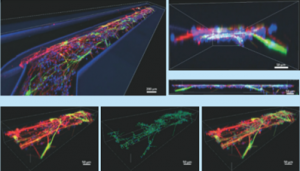 Differentiation of Human Neuroepithelial Cells in Organ Chip Microchannels
Differentiation of Human Neuroepithelial Cells in Organ Chip Microchannels
ALP activity detection methods include but are not limited to:
- Calcium cobalt method
When the pH is 9.4, magnesium ions are used as an activator to hydrolyze the naturally occurring sodium β-glycerophosphate to release phosphate radicals, which combine with calcium ions to form a precipitate, and then react with cobalt nitrate to form cobalt phosphate, which is treated with amine sulfide to form a black cobalt sulfide precipitate. The method is a stop-staining method that uses metal precipitation to reveal alkaline phosphatase activity.
- AMP method
The method uses p-nitrophenol phosphate (4-NPP) as the substrate and 2-amino-2-methyl-1-propanol (AMP) as the phosphoryl acceptor substance (enhancing the enzyme reaction rate). After being catalyzed by ALP, 4 -NPP cleaves a phosphate group to generate free p-nitrophenol (4-NP), which transforms into a quinone structure in an alkaline solution and presents a darker yellow color. And the alkaline phosphatase activity can be calculated by measuring the rising rate of the molar extinction coefficient at 405nm.
- Disodium phenyl phosphate method
When the pH is 10, ALP can catalyze the hydrolysis of disodium phenyl phosphate to phenol and disodium hydrogen phosphate. Phenol reacts with 4-amino antipyrine, and is oxidized by potassium ferricyanide to generate quinone derivatives. The alkaline phosphatase activity can be calculated by measuring the absorbance at 510nm by colorimetry.
- BCIP/NBT colorimetric method
BCIP/NBT (nitro blue tetrazolium) is a substrate of alkaline phosphatase. Under the catalysis of alkaline phosphatase, BCIP is hydrolyzed, and the hydrolyzed product reacts with NBT to form an insoluble dark blue to blue-purple product.
- Angiogenesis Assay
Angiogenesis is a physiological process that primarily refers to the formation of new blood vessels that develop from existing capillaries or postcapillary veins. Furthermore, angiogenesis also plays an important role in pathological conditions such as inflammation, degenerative diseases and tumor formation. The organ-on-a-chip model can simulate the physiological response of the organ system in the human body, and provides a new platform for understanding the biological process of angiogenesis and the treatment of diseases in which angiogenesis is involved.
Angiogenesis assays include, but are not limited to:
- Endothelial cell migration assay
Commonly used methods include the Boyden Chamber method, in which cells are placed in the upper compartment, and allowed to migrate through membrane pores to the lower compartment with chemoattractants placed in the lower compartment. After a period of incubation, the membrane between the two compartments is fixed and stained to determine the number of cells that have migrated to the underside of the membrane.
- Endothelial cell proliferation assay
A commonly used method is MTT colorimetry, The principle is that succinate dehydrogenase in the mitochondria of living cells can reduce exogenous MTT to blue-purple crystal formazan and deposit in the cells, while dead cells do not have this function. Dimethyl sulfoxide (DMSO) can dissolve formazan in cells, and its light absorption value is measured at a wavelength of 570nm with an enzyme-linked immunosorbent assay. Within a certain range of cell numbers, the amount of MTT crystal formation is proportional to the number of cells.
- Tube formation assay
In the culture medium containing collagen and fibrin, the endothelial cells initially formed a cell cord structure, and after a period of culture, the cell cord began to branch and penetrate the gel to form a three-dimensional tubular network structure. Tube formation determination involves taking pictures of tubes of different lengths from top to bottom, and using software to measure the length and maximum diameter of each tube.
Reference:
[1] Liu H T; et al. Advances in Hydrogels in Organoids and Organs-on-a-Chip[J]. Adv. Mater. 2019, 1902042.
[2] Castiello F R; et al. Microfluidic perfusion systems for secretion fingerprint analysis of pancreatic islets: applications, challenges and opportunities[J]. Lab on a Chip. 2016, 16(3): 409-431.
[3] Lee J; et al. Fabrication and characterization of microfluidic liver-on-a-chip using microsomal enzymes[J]. Enzyme Microb. Technol. 2013, 53(3): 159–164.
[4] Moreno E; et al. Differentiation of neuroepithelial stem cells into functional dopaminergic neurons in 3D microfluidic cell culture[J]. Lab on a Chip. 2015, 15: 2419-28.
Related Services: