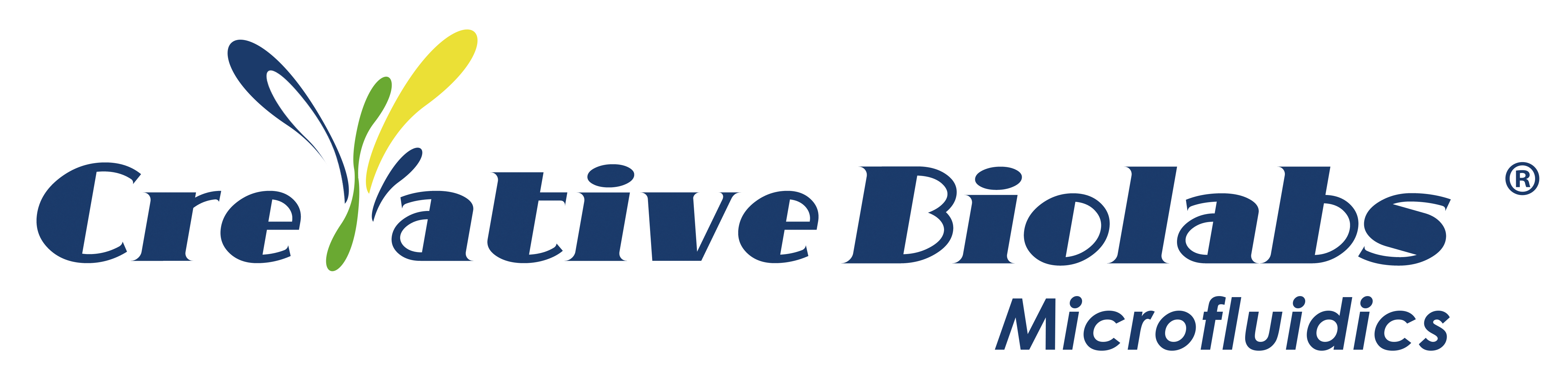High-quality preclinical bioassay models are critical for drug development. Organ chips replace living organisms by bionically constructing micro-organs on microfluidic chips for drug evaluation and biological research. The organ-on-a-chip model can control the cellular microenvironment and simulate the real and complex physiological processes in the human body. It is an ideal model for predicting preclinical drug efficacy, metabolic transformation, and drug toxicity determination.

As a company specialized in organ chip R&D services, Creative Biolabs provides comprehensive and high-quality organ chip-based detection services, which can simulate different in vivo conditions for in vitro experiments, observe the growth of different cells at different times and spaces, and detect the corresponding indicators.
Detection Technology Based on Organ Chips
Pharmacokinetic/pharmacodynamic modeling
A pharmacokinetic model is an important tool in pharmacological research, mainly to study the in vivo process of drugs, the effect of drugs on the body, and the relationship between them, and it is widely used in the fields of describing drug research, development, and use. The application of pharmacokinetic/pharmacodynamic models in drug research can provide a scientific basis for elucidating the material basis of drug efficacy and mechanism of action, such as the evaluation of drug effects, optimization of drug regimens, and providing evidence for clinical medication.
- PBPK model
Physiologically Based Pharmacokinetic (PBPK) is a simulation model for predicting the absorption, distribution, metabolism, and excretion of substances in organisms. It is used in the fields of pharmacokinetics of drugs and exposure assessment of environmental toxicants. This model replaces the compartments in the classic model with “physiological compartments”, which represent single or multiple organs, tissues, or body fluids that are mainly related to the distribution of drugs in vivo. The drug enters a “physiological chamber” through various biofilms with the blood flow, and may be eliminated when it leaves the “physiological chamber”, which is described by various clearance rates of the chamber (metabolic clearance rate, excretion clearance rate). According to the mass balance relationship, the velocity equation is established according to the model, and the equations are solved to obtain the relationship between the concentration of the poison in each tissue or organ and the time.
 Organs important for ADME are included in the PBPK model
Organs important for ADME are included in the PBPK model
- CFD model
Computational Fluid Dynamics (CFD) is the product of the combination of modern fluid mechanics, numerical mathematics, and computer science. The integral and differential terms in the governing equations of fluid mechanics are approximately expressed as discrete algebraic forms, making them into algebraic equations, and then these discrete algebraic equations are solved by computer to obtain numerical solutions at discrete time/space points. It is mainly used in the design of drug delivery systems and the analysis of drug absorption.
Proliferation Assay (EdU Assay)
Cell proliferation assays can be used to determine the division status of cells and to quantify expanded cell populations, which are critical to cell viability and function analysis. However, the method for detecting cell proliferation is very cumbersome and requires the use of radioactive molecule 3H-thymidine integrated into the genetic material of new progeny cells for judgment. Organ chip technology can maximize the effect of extracellular matrix on cell proliferation, analyze cell proliferation by detecting changes in intracellular markers or DNA, and study immune phenotypes, compound toxicity, effects of drugs on tumor cell growth, cell behavior under different conditions, etc.
Cell proliferation detection techniques include but are not limited to:
RT-qPCR array technology: SYBR Green dye-based detection method for reliable gene expression analysis of 41 genes involved in several pathways related to DNA damage response, cell cycle progression, cellular senescence, and programmed cell death.
ELISA proliferation assay (colorimetric method): Use ELISA colorimetric detector to detect cell proliferation. The absorbance value in the experimental results is directly related to DNA synthesis, that is, directly related to cell proliferation.
ELISA proliferation assay (chemiluminescence method): use an ELISA luminescence detector to detect cell proliferation. As a result, the relative light unit/second (rlu/s) is directly related to the amount of DNA synthesis, and the number of proliferating cells can be detected in each microplate.
EdU analysis: EdU (5-ethynyl-2′-deoxyuridine) is an analogue of thymidine, which can be incorporated into replicating DNA molecules during cell proliferation, and can be quickly detected by combining EdU with specific fluorescent dyes DNA replication activity is detected to measure cell proliferation.
Induced apoptosis assay
Cell apoptosis refers to the process of actively ending the life of cells controlled by internal genetic mechanisms in order to better adapt to the living environment under certain physiological or pathological conditions. “Apoptosis” is a description of a series of morphological changes in a fixed pattern during cell death. Creative Biolabs offers a variety of organ chips to induce apoptosis for the evaluation of target drugs. The commonly used analysis method for inducing apoptosis is to integrate the quantitative data acquisition method with the chip platform, and perform imaging analysis on cells by optical detection method.
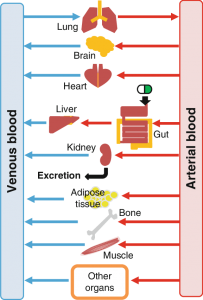
 Application of High Content Confocal Imaging in Organ Chips (Including Apoptosis Analysis)
Application of High Content Confocal Imaging in Organ Chips (Including Apoptosis Analysis)
Annexin V (Annexin V) is a Ca²⁺-dependent phospholipid-binding protein that can bind highly specifically to phosphatidylserine (in the early stage of apoptosis, phosphatidylserine can flip from the inner side of the cell membrane to the surface of the cell membrane, Exposure to the extracellular environment), which can be used as a probe to distinguish early apoptosis, late apoptosis, and dead cells. Other induction methods, such as the combination of benzbromarone (whose mechanism of toxicity is associated with mitochondrial damage and subsequent induction of apoptosis and necrosis) and staurosporine (a tool inducer of apoptosis) have been used successfully in the evaluation of the hepatotoxic effect of candidate drugs, and they have good adaptability to kidney organ chips of different shapes and sizes.
Photobleaching Fluorescence Recovery Technology (FRAP)
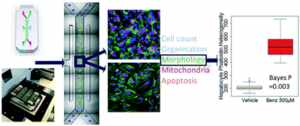
Fluorescence recovery after photobleaching (FRAP), which uses fluorescent substances to mark cell membrane proteins or membrane lipids, irradiates a certain area of the cell with high-intensity pulsed laser light, causing the light quenching of fluorescent molecules in this area. If the membrane protein is flowing, when the laser irradiation is stopped, the fluorescence at the bleached site can be restored, and the diffusion rate of the membrane protein or membrane lipid can be calculated according to the recovery speed. This approach allows researchers to monitor protein trafficking in individual cells or even individual gas chromatography columns with subsecond precision.
 Schematic diagram of FRAP detection experiment
Schematic diagram of FRAP detection experiment
The integration of organ chips and a variety of embedded sensors can realize long-term automated monitoring and screening of drug effects. In FRAP detection, it is connected in series with the organ chip to realize real-time monitoring of cells, which is highly flexible and operable in the detection of different organ chips. For different cell types, Creative Biolabs will tailor different solutions based on rich project experience and customer needs. For example, for rapidly diffusing cells, the image acquisition rate can be increased by changing the scan speed or reducing the size of the overall frame to be acquired, or increasing the size of the region of interest (ROI) for bleach, optionally increasing the laser power and other methods to obtain optimized images and results.
References:
[1] Hyun K S; et al. Predicting ADME Properties of Chemicals. Handbook of Computational Chemistry. 2016, 1-37.
[2] Peel S; et al. Introducing an automated high-content confocal imaging approach for Organs-on-Chips. Lab on a Chip. 2019, 19(3): 410-421.
[3] Xiong, R.; et al. Sizing nanomaterials in bio-fluids by cFRAP enables protein aggregation measurements and diagnosis of bio-barrier permeability. Nat Commun. 2016, 7: 12982.
Related Services: