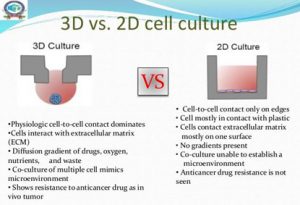
Over the past few years, two-dimensional (2D) monolayer cell cultures have been the main experimental model for drug discovery, however, for some applications, monolayer cell cultures are situations where cells proliferate and grow in altered in vitro environments, which could cause the loss of original tissue traits. Therefore, it is difficult for 2D cell culture to properly reflect the in vivo growth environment of cells. In vitro three-dimensional (3D) culture provides cells with a microenvironment that is closer to the living conditions in vivo, which can better simulate the physiological state, enable cells to express reasonable genes and traits, and have the advantages of intuitive observation and controllable conditions. Therefore, 3D cell culture is increasingly used in basic research and drug discovery.
Scaffold-based 3D cell culture system
ECM scaffold method
The extracellular matrix (EMC) scaffold method is one of the most commonly used techniques in 3D culture. Adult stem cells are first coated on a commonly used ECM protein mixture gel matrix and maintained under culture conditions. When specific cell types are desired, cells are then stained with antibodies, sorted using a fluorescence-activated cell sorting (FACS) system, and plated on individual gel matrix dishes. The advantage of this method is that it can monitor cell biological processes (such as cell adhesion, migration, and chemotaxis) in a tissue-like environment. The disadvantage is that the reproducibility is not high, because different hydrogel production batches have different mechanical properties, which will affect the formation of organic matter.
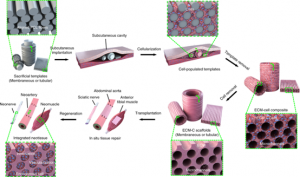
Schematic diagram of the generation and application of the ECM scaffold
Artificial matrix scaffold
There are quite a few types of synthetic artificial matrix materials, such as biomimetic hydrogel materials, polystyrene scaffold materials, and nanofiber materials. Cells can be embedded in hydrogels containing cross-linking agents in the form of single cells or cell clusters. If the cultured cells produce the corresponding extracellular matrix metalloproteinases, they can cleave adjacent matrix cross-linking agents. Corresponding cell adhesion factors could be added to induce cell spreading and migration. The disadvantages include difficulty to separate cells from the scaffold, the high cost of culture, and difficulty in a large-scale application.

Polymer hydrogels for 3D cell culture
Scaffold-free 3D cell culture system
Rotary bioreactor method
This method mainly uses the principle of suspension culture, where the rotating container drives the cells to suspend and rotate, and the cells aggregate into clusters during the continuous rotating motion. The method is simple to operate and easy to form into balls, and the exchange of nutrients between cells can be promoted by agitation, allowing for large-scale cultivation. Disadvantages include the production of spheroids with widely varying sizes, and the potential for shear forces experienced by cells in the bioreactor to affect cell physiology.
Hanging drop method
Cells self-assemble into microspheres under the action of gravity by hanging drops. The microtissue sphere can be precisely controlled by controlling the hanging drop, so that it has high consistency and provides a good microtissue material for follow-up research. Moreover, the co-cultivation of different cell types can be realized by the hanging drop method to ensure information exchange between the co-cultured cells. The disadvantages include the high cost of liquid handling robotics, the limited droplet size, and the inability to change medium midway.
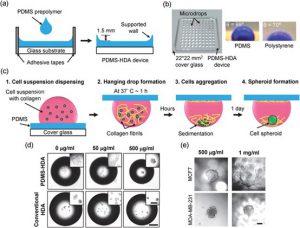 3D spheroid culture in PDMS-based hanging drop array
3D spheroid culture in PDMS-based hanging drop array
Low Adhesion Plane Method
The specially treated low-adhesion culture plate can reduce cell adhesion, protein absorption, and enzyme activity as much as possible, maintain the growth of cells in a non-adherent state, and make the cultured cells agglomerate and form spheroids. This technique produces larger spheroids and is cost-effective, but it has the disadvantage of not being applicable to certain cell types and often requiring additional experimental steps.
Magnetic Levitation
The method involves first incubating cells overnight with phage, polydisperse gold nanoparticles, and magnetic hydrogel-coated iron oxide nanoparticles. The nanoparticles are then taken up by the cells and digested with trypsin. Then place cells on a low-attachment plate and place the magnetic lid on top to attract nanoparticles and generate a liquid-air cell suspension. The advantages of this method are the fast growth of spheroids, no need for special substrates, and reproducible necrotic and hypoxic regions, but the disadvantages are that nanoparticles are expensive and potentially cytotoxic.
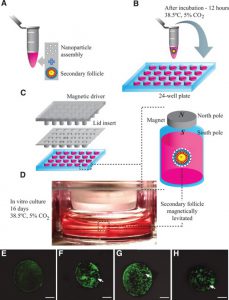 Schematic diagram of magnetic levitation 3D cell culture
Schematic diagram of magnetic levitation 3D cell culture
Bioprinting
The principle of this method is derived from 3D printing technology, which uses a bioprinter to accumulate biological materials layer by layer, and finally achieves the required complexity structure. The advantage of this technology is that it can achieve accurate and versatile printing of multiple components, which can be customized, while the disadvantage is the requirement of expensive printing equipment and bio-inks with the required properties and viscosity.
Microfluidics and 3D Cell Culture
At present, the combination of microengineering, microfluidics, and 3D cell culture to develop a perfusion 3D culture system (also known as a microfluidic chip) has become a hot field of 3D cell culture research. Dynamic perfusion culture is more advantageous over a static culture system because cells can fully access fresh nutrients and oxygen. With the advantages of high throughput, integration, and low reagent consumption, microfluidic chips have become an important platform for the development of new-generation drugs.

Reference:
[1] Zhu M; et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration[J]. Nat Commun. 2019, 10: 4620.
[2] Tong CQ; et al. Photopatternable, Branched Polymer Hydrogels Based on Linear Macromonomers for 3D Cell Culture Applications[J]. ACS Macro Lett. 2020, 9, 6: 882–888.
[3] Kuo, CT; et al. Three-dimensional spheroid culture targeting versatile tissue bioassays using a PDMS-based hanging drop array[J]. Sci Rep. 2017, 7: 4363.
[4] Deize C A; et al. Three-dimensional levitation culture improves in-vitro growth of secondary follicles in bovine model[J]. Reprod Biomed Online. 2018, 38, 3:300-311.
Related Services:
CBLMF™ 3D Tissue and Organ-On-A-Chip Model Development Services
Cell Culture and Organ-On-A-Chip Model Development Services