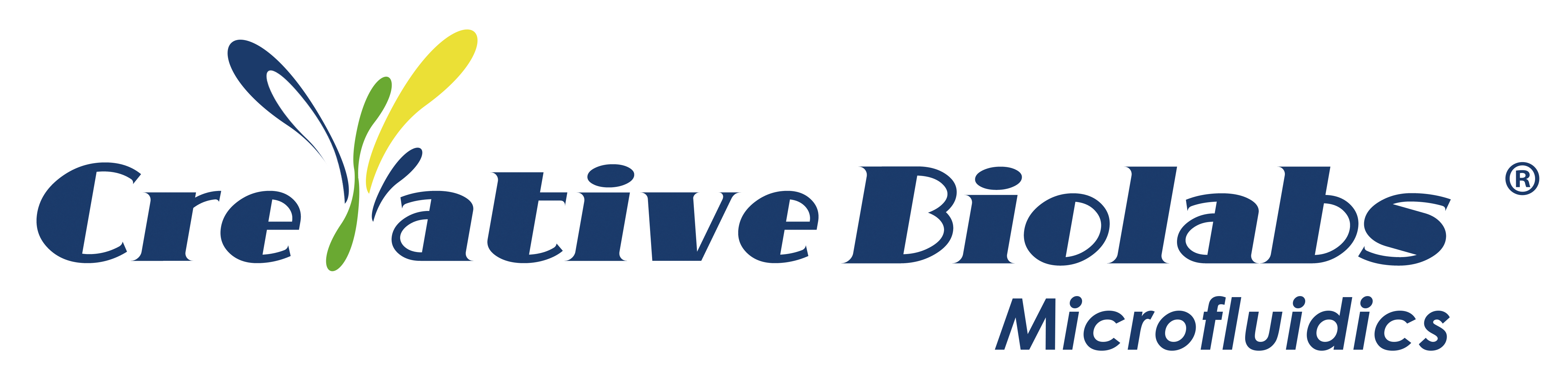Recently, an article titled Complex 3D microfluidic architectures formed by mechanically guided compressive buckling was published on Science Advance and became the cover of the current issue.

- This research has taken a big step towards the realization of intelligent artificial microvascular networks.
According to the author, this research not only expands new boundaries of knowledge, but also solves the problems and challenges that existed before. The subject of this research is a complex three-dimensional microfluidic network formed by mechanically guided compression and buckling.
On the one hand, he expanded the boundary of mechanics-guided three-dimensional micro-scale assembly method in the preparation of three-dimensional microstructure composed of soft materials (the elastic modulus of the materials used in the study was in the order of 100kPa~1MPa); on the other hand, he solved two difficult problems: the ultra-small flow channel size and complex shape of the three-dimensional microfluidic control equipment, and meanwhile demonstrated the ability to easily integrate electronic devices on the three-dimensional microfluidic system.
As a technology that can accurately manipulate micro-scale fluids, microfluidics can quantitatively transport liquids and gases through micro-sized fluid channels. It plays an important role in chemical analysis, drug delivery, artificial blood vessels, and other fields.

Because it can achieve a larger range of nutrient delivery, the artificial blood vessel network based on microfluidic technology has clear significance in realizing larger-scale three-dimensional cell culture, regenerating tissues, and artificial organs.
However, even if the prospects are bright, many methods for preparing complex three-dimensional microfluidics, such as 3D printing, have faced certain limitations before. Typical challenges are difficulties of forming complex and controllable three-dimensional shapes, reaching a size of flow channel that is equal to or smaller than capillaries (about 10 microns in diameter), having a transparent geometric configuration, realizing a fast and high-throughput manufacturing process, and integrating high-performance electronic devices for sensing and actuation, and other challenges. But the methods shown in this study are not subject to the above restrictions.
In the research, the author used the method of compression buckling to assemble the two-dimensional microfluidic flow channel into a three-dimensional microfluidic network in a controllable manner, thus completely overcoming the above-mentioned difficulties.

Based on the mature planar micro-nano processing technology, he demonstrated the 3D microfluidic design with 4 microns and even less flow channel width in the research. While maintaining the small flow channel size, he also realized complex geometric shapes and a larger coverage area, having expanded the achievable geometric shape and working characteristics of the artificial microvascular network.

The author stated that the method used in this study can effectively control the shape and size of the three-dimensional geometry, as well as the fluid flow rate, pressure distribution, and the mechanical properties of the structure. The overall design freedom is relatively high.
In addition, in terms of design, the three-dimensional microfluidic system is more “complex” and complicated, which also gives it a lot of capabilities, such as the fluid, thermal, and electromagnetic properties that can be used to control the system. Since the three-dimensional combination shape is relatively transparent, it can also be conveniently used to integrate various biological & non-biological materials.
- Inspired by animal blood vessels, through eight major research steps
As a lot of scientific research will draw inspiration from the animals and plants in nature, this time the author draws inspiration directly from blood vessels.
He said that the initial inspiration for the research came from the vascular network. The blood vessel network in organisms is complex, not only with multiple levels and branches but with different sizes at each level. The blood vessels and heart in vertebrates constitute a complete blood circulation system. The blood vessels can be further divided into arteries, arterioles, capillaries, venules, veins, and more.
Take the human pulmonary artery as an example, it is composed of over 15 branch orders. The vascular network can provide oxygen, nutrients, water and other important substances to the cells in the body, and can take away metabolic waste; the capillary network can cover all parts of the body to ensure that the whole body’s cells are within the range of blood circulation.
To imitate the complex biological vascular network and combine it with the three-dimensional assembly technology of Luan Haiwen’s team, the first step is to expand applicable materials for the three-dimensional assembly technology. It is theoretically proved that the three-dimensional assembly method is suitable for various hard and soft materials, and is suitable for multiple geometrical scales of micro, mesoscopic, and macroscopic.
Because biological blood vessels are relatively soft, he also used a soft, transparent, and biological tissue-compatible silicone material—polydimethylsiloxane (PDMS) to prepare the microfluidic system.
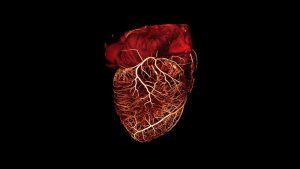
The second step is to solve the key mechanics problem. Specifically, the author needed to avoid the adhesion between the microfluidic structure and the elastic substrate and the collapse of the flow channel due to buckling deformation through the mechanical analysis and design of the system during the three-dimensional assembly process.
And by establishing and optimizing the finite element analysis model, he was able to accurately predict the shape and stress distribution of the three-dimensional structure, so as to improve and optimize the design.
The third step is to explore complex geometric shapes. Combining simulation and experimental attempts, the author made more than ten different geometric shapes of three-dimensional microfluidics, thereby enriching the design library of a single microfluidic structure.
The fourth step is to build a microfluidic network array. That is, the microfluidic networks of different sizes and shapes are connected together to form a more complex microfluidic system. It also involves the design of remotely conveying fluids for different three-dimensional structures through the flow channels on the elastic substrate, similar to the blood flowing out of the heart and branching through many organs and tissues, and then returning to the heart.
The fifth step is to explore the size limit, including the size of the flow channel, such as a few microns in width or height, as well as the size of the structure outline. It is currently possible to effectively control the laser cutting of the structure outline at about 50 microns outside the flow channel. In addition to the above, the overall size of the three-dimensional microfluidic structure is not too limited.
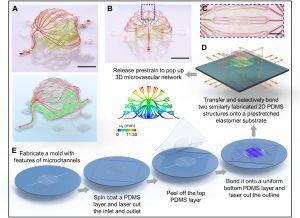
The sixth step is to increase the display of three-dimensional microvessels in bionics, including matching with the size and configuration of the human blood vessel network. For example, the case displayed on the cover of the journal consists of two stacked structures, each of which has three levels of branches, and the flow channel width of them respectively is 100 microns, 30 microns and 10 microns. The size of 10 microns is similar to the diameter of capillaries in the human body. The flow channel took advantage of the air permeability of PDMS film to achieve stable oxygen delivery, and the porous PDMS film is used to make the flow channel wall for macromolecular protein transportation.
The seventh step shows various integrated high-performance electronic functions, which is also the unique advantage of this three-dimensional microfluidic structure. The multifunctional microfluidic-electronic three-dimensional combined system shown in the article represents a new generation of intelligent three-dimensional microvascular network with electronic sensing and executive functions as well as fluid transport functions.
In the system array structure, various electronic devices are located directly above the microfluidic flow channel. The electronic devices include 16 groups of uniformly distributed micron-level inorganic LED light sources and thermistors (or heating resistors), as well as 12 microelectrodes, which can be used for light stimulation, heating, electrical stimulation, temperature sensing, electrical signal acquisition, etc. Each electronic device can be controlled independently.
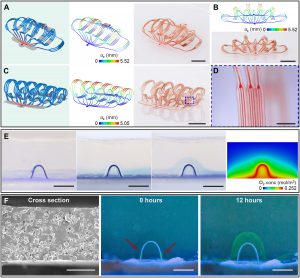
The soft and malleable mechanical properties enable the microfluidic-electronic three-dimensional combination system to withstand bending, torsion, stretching and other deformations. The research showed the case of wrapping the devices around a glass rod. Mechanical analysis and design ensure that the gold wire in the flexible circuit of the three-dimensional bonding system is always in the elastic range during the three-dimensional assembly process, ensuring that the device has reliable electronic functions.
In the eighth step, in order to prove the wide applicability of buckling assembly, the author demonstrated an example of using buckling assembly to transform an irregular two-dimensional microfluidic configuration into a three-dimensional complex network through simulation and experimental methods.
- Can be used for in vitro tissue culture, nutrient delivery for artificial organs, etc.
In terms of application prospects, the author stated that the three-dimensional artificial microvascular network made this time integrates fluid channels with high-performance electronic devices, and has multiple functions such as fluid transport, fluid sensing, and photothermoelectric stimulation.
In the research, he tried to increase the complexity and completeness of the three-dimensional artificial microvascular network to achieve functions similar to the biological blood vessel network.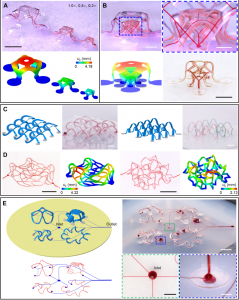
Specifically, the three-dimensional artificial microvascular network can be used for tissue culture in vitro, and can also be used to deliver nutrients and discharge waste for artificial organs.
Compared with the traditional two-dimensional microfluidic system, it can help cultivate more complete functional tissues and organs for basic medical and biological research, such as in vitro diseases and blood flow characteristics.
Using the embedding method, the three-dimensional artificial microvascular network can also help cultivate larger and more powerful muscle tissue for use in biological hybrid robots.
In the future, the author will continue studying the assembly of three-dimensional micro-scale structures and bio-integrated electronics, and strive to make contributions both in theory and experiment, and hope to continue the research on microvascular networks.
Reference:
Haiwen Luan, Qihui Zhang, et al., Complex 3D microfluidic architectures formed by mechanically guided compressive buckling. Adv. 7, eabj3686 (2021)