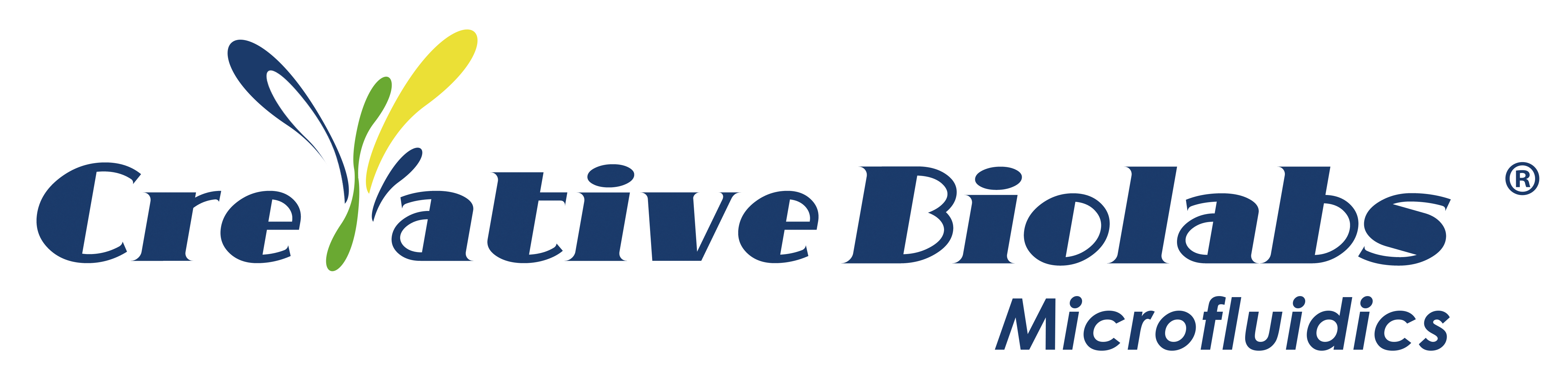Organ-on-chips (OOC), also known as microphysiological systems or “tissue-on-chips”, have received increasing attention in recent years due to their potential to provide useful information during drug discovery and development. Organ-on-a-chip is broadly defined as a miniature cell culture device used to simulate the functional units of human organs in vitro. It simulates the biological environment of cultivating cells, tissues, and organs on chips to study and control the biological behaviors of cells in vitro, thereby achieving the goals of organ transplantation and drug evaluation under a simulated biological environment.
Organ-on-a-chip production background – new drug discovery dilemma
The long life cycle, large investments, and low success rate are the current difficulties faced by drug research and development. The Tufts Center for the Study of Drug Development estimated the cost of a drug all its way from the lab to the market in the U.S. is about $2.7 billion. Another analysis of data based on 10 cancer drugs showed that the median cost of a single drug from the lab to the market was about $648 million. However, pharmaceutical companies have not been successful in licensing most cancer drugs, and many drug candidates ultimately are unable to enter the market or withdrawn due to toxicology and safety concerns, or due to unforeseen circumstances that occurred during preclinical and clinical trials (figure 1).
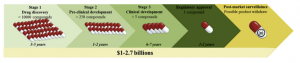
Fig.1 Drug Development Process
On the other hand, preclinical development of drugs often involves testing in tumor cell cultures as well as animal models. Traditional 2D cell cultures have been used for more than 50 years to assess the effects of drugs on tumor cell growth, however, they fail to provide insight into complex interaction information between cancer cells, associated stromal components, and the physicochemical microenvironment that exists within living tumors of human organs. Conventional 3D cell culture hydrogel matrices can provide a three-dimensional culture environment, but still cannot controllably reproduce in vivo physiology and human pathology. Animal experiments often involve subcutaneously implanting tumors in rodents, but these models are widely considered poor models because they lack the characteristics of tissue-specific microenvironments. As a result, researchers began to xenograft human tumors into the same organ site in mice, and these in vivo orthotopic tumor models have been shown to better mimic tumor growth and metastasis2,3. However, orthotopic mouse models are still unable to recreate the human tumor microenvironment, which is a key factor in tumor growth and progression. In addition, the animal’s response to the drug is different from the human response due to species differences and other reasons. These in turn lead to the lack of accuracy and predictability of drug results in animal experiments, making human clinical trials of drugs face a greater risk of failure.
Despite significant advances in computational biology, in vitro biology, and toxicology over the past 20 years, more than 80% of clinical trial drugs currently fail clinical trials, 60% of which are due to lack of efficacy, and another 30% is due to toxicity issues. To address these issues and provide an effective alternative tool for the preclinical stage, organ-on-a-chip has emerged (Figure 2).

Fig.2 Advantages of microfluidic organ-on-a-chip platforms
Composition and main features of organ-on-a-chip
Organ-on-a-chip is made up of multi-disciplinary technologies. It is mainly based on microfluidic chip technology, which integrates with technologies such as micromachining, stem cells, materials, and biological tissue engineering. It includes a variety of living cells, functional tissue interfaces, and biological fluids, with physiological functions close to the human level and the ability to precisely control multiple system parameters. Researchers can more intuitively study body behavior and predict or reproduce the effects of drugs, toxins, radiation, cigarette smoke, pathogens, and the normal microbiome on the human body.
Organ chips are microfluidic cell culture devices composed of transparent plastic, glass, or flexible polymers such as polydimethylsiloxane (PDMS). Its essence is a three-dimensional cell culture system based on a multi-channel fluidic chip, which is composed of multiple cell culture partitions that simulate the human tissue and organ environment, and the partitions are connected by a bionic circulatory system (Figure 3). It’s called a “chip” because the microfabrication method it originally used was an improvement on the fabrication of computer microchips. In addition, the organ-on-a-chip also contains integrated miniature sensing and imaging devices for real-time, online detection of the growth microenvironment and state of three-dimensional cell aggregates, and the interaction between tissues and organs. It reproduces the physiological and pathological features at the organ level in vivo by reconstructing structure and function at the tissue and organ level in vitro.

Fig.3 Multi-organ microfluidic framework
The main feature of an organ-on-a-chip is a bioengineered microdevice capable of recapitulating key functions of organs and tissues. The specific design of each platform varies, and organ-on-a-chip specifications range from USB thumb drive sized devices to more complex systems capable of mirroring multiple connected organs. All OOC platforms share three key features: 1. The 3D nature and arrangement of the tissues on the platform, which is usually achieved by adding biocompatible materials such as hydrogels to help prevent mechanical damage. 2. Presenting and integrating multiple cell types to reflect the physiological balance of cells (e.g., parenchymal cells, interstitial cells, vascular cells, and immune cells). 3. Presenting biomechanics relevant to modeled tissues, such as the stretching force of lung tissue and the hemodynamic shear force of vascular tissue. Introducing biomechanics into tissues to simulate fluid flow and adding microfluidic channels into the system can deliver and remove cell culture media, and to remove associated cellular metabolites and debris. In addition, some platforms also require stimulation or drug delivery equipment, and for some tissues, physical or chemical signals are required to simulate the physiological microenvironment and promote microtissue maturation. For example, electrical stimulation can help myocardial tissue mature. Different signal stimuli can be used for drug screening. The sensing component for data detection and compilation can be an embedded sensing output component or a transparent chip-based image analysis function evaluation system.
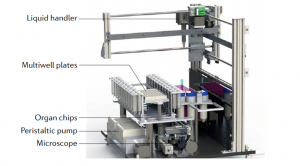
Fig.4 Automated System for Organ-on-a-Chip
The construction and design of a specific organ chip are based on the miniaturization of the target organ. From the anatomical point of view of the target organ, its structure and function are decomposed into specific units and elements to be realized in vitro, such as selecting the cell type for constructing in vitro organoids, reconstructing the 3D structure of cell culture, and replicating the organ-specific physiological environment. In order to better reproduce the characteristics of the target organ, organ-on-a-chip usually designs multiple microchambers for cell and tissue culture, and each microchamber has a separate microfluidic channel for supplying nutrients and expelling metabolites to the organoids, along with additional components such as mechanical drive, optical imaging, transmembrane resistance measurement, and biochemical detection modules. Taking the lung organ chip as an example (Fig. 5), in order to simulate the relaxation of alveoli and capillaries during respiration, the micro-vacuum chambers on both sides of the chip induce the stretching of the cell lining membrane through the action of circulating vacuum, the frequency and intensity of action of which are similar to human breathing rates8. The same device and method are also applied to the peristalsis of small intestinal villi in a pseudo-gut chip.
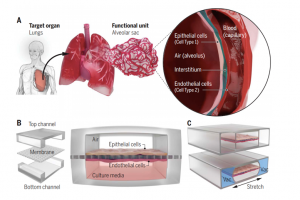
Fig.5 Schematic diagram of the design of lung organ-on-a-chip
Advantages of Organ-on-a-Chip
A key advantage of the organ-on-a-chip platform compared to traditional 2D static cell culture methods is the ability to control cells and specific tissue structures to simulate chemical gradients and biomechanics, allowing precise control of the biochemical and cellular environment, mimic of the in vivo environment and responses, as well as high-resolution and real-time imaging and in vitro analysis of the biochemical, genetic, and metabolic activity of living human cells in the context of functional human tissues and organs. In addition, the organ-on-a-chip has tissue vascularization and perfusion capabilities, including self-assembling endothelial cells to form perfusion lumens, or using microfluidic channels as engineered vascular systems to deliver nutrients and fluids to cells in culture chambers. It can also incorporate real-time tissue function sensors, such as microelectrodes and light microscopy markers like fluorescent biomarkers, allowing monitoring of cell health and dynamic activity.
In addition, organ-on-a-chip is also more cost-effective than traditional animal testing. Although animal models have made enormous contributions to understanding physiology and disease and developing new drugs, researchers have long been aware of the frequent inconsistencies between animal and human studies and the prohibitive costs of doing so. According to reports, the cost of animal testing is about $6,500-800,000 depending on the length and complexity of the experiment, and these figures do not take into account the cost of raising multiple animals for animal research institutions. An internal report by the European Organ-on-a-Chip Association showed that in hepatotoxicity studies, the experimental cost of OOC was 10% of that of animal experiments, and the cycle was shortened by 90%.
Main applications of organ-on-a-chip
Organ-on-a-chip plays an increasingly prominent role in disease research, new drug development, toxicity evaluation, and other fields, providing a systematic solution for life science and medical research. Organ-on-a-chip is currently mainly used in the field of drug discovery, and has begun to partially replace animal experiments, becoming one of the most valuable tools in the fields of preclinical drug development, medical devices, environmental risks, nutrition, and biomaterials. Meanwhile, Organ on chips are also used to screen beneficial or harmful chemicals, functional materials, food ingredients, and consumer products. In addition, organ chips can also generate in vitro organ models or even disease models of individuals or special populations, which can help the fields of precision medicine and precision nutrition.
1. Toxicity assessment
To assess the toxicity of drugs to organs, cell culture and animal models are often used for analysis, but they cannot replicate the complex system responses of drugs in the human body, nor can they accurately predict the effects in humans. Therefore, various unexpected problems are very likely to occur in the process of translation of research results, especially during the first phase of clinical trials. When the drug “enters the human body for the first time” or the dose is increased, volunteers in clinical trials can be easily put in a high-risk situation. On the basis of fully understanding the complex tissue and organ structure and physiological function characteristics of the human body, the organ chip provides a near-physiological in vitro model for drug research and development, disease research, chemical, toxin and cosmetic testing, which has a wide application value in several fields.
2. Disease modeling
Organ-on-a-chip can also simulate multiple types of organ-specific disease states in vitro, including Barth syndrome, pulmonary edema and thrombosis, asthma, chronic obstructive pulmonary disease, and inflammatory bowel disease, which allows mechanism research on disease pathology, the efficacy of therapeutic interventions, and potential off-target effects, effectively reducing the failure rate in the clinical development stage. In recent years, researchers have gradually introduced pluripotent stem cell technology into the field of organ-on-a-chip and have successfully established a variety of disease models. For example, a team collected stem cells from patients to build a functional cardiac tissue chip, which successfully simulated Barth syndrome, a form of childhood cardiomyopathy.
3. Drug Evaluation
Drug evaluation mainly studies the functions and rules between drugs and the human body and determines their effectiveness and safety according to the in vivo process of drug absorption, distribution, metabolism, and excretion (ADME). The organ chip can reflect the dynamic changes of the drug in the body and the real response of human organs to drug stimulation, which can make up for the large deviation between the existing model and the human body, and constitute a three-in-one druggability evaluation technology system for pharmacokinetics, efficacy, and toxicity, helping researchers intuitively perceive and evaluate the safety and effectiveness of new drugs.
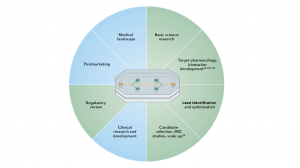
Fig.6 Organ-on-a-chip can be used in all aspects except clinical trials and drug sales
At present, many companies in the pharmaceutical, cosmetic, and consumer products industries are using their organ chips for testing, including efficacy and safety testing of new drugs and other products. For instance, L’Oreal, Pfizer, AstraZeneca, Roche, Sanofi, Hengrui, Pharma Ming Kangde and others have already begun to cooperate with organ-on-chip research and development institutions.
Development pain points of organ chips
1. Cell Source
A common problem faced by developers of organ-on-a-chip is the procurement of regenerative cells, and the selection of suitable cell systems is based in part on the platform used and availability. Cell systems are now increasingly choosing iPS cells or adult stem cells from tissue organoids9. iPS cells have many advantages, for example, iPS cells provide a nearly unlimited source of cells, and the generation of isogenic cell lines from them means that all tissues in a cell platform may be derived from the same donor. However, to date, many iPS cell-derived differentiated cells, such as cardiomyocytes, are immature in phenotype, and the differentiation and maturation are not standardized, which can lead to difficult replication.
2. Manufacturing materials
Another potential factor affecting the rapid development of organ-on-chips is the raw material used to manufacture organ-on-chips, polydimethylsiloxane (PDMS). PDMS is a silicon-based organic polymer widely used in chip fabrication because it is inexpensive, easy to use, allows rapid prototyping and easy iterative design changes through soft lithography methods, and at the same time, it is biocompatible and optically transparent, which allows biomechanical modeling and real-time tissue imaging. However, PDMS is gas permeable and has high absorbance for small hydrophobic molecules. Therefore, PDMS still has some problems for drug research. For example, PDMS itself may absorb a large number of drugs, or some factors released by cells may seep out of wastewater, resulting in cross-contamination of adjacent chambers or channels. risk. Currently, mitigation methods include treating or coating the polymer surface of the device to prevent cell adhesion or drug loss. But to unlock the full potential of organ-on-a-chip, new alternative materials still need to be found that are flexible and transparent, while keeping the absorption of drugs and cellular nutrients to a minimum. At present, the academic community has carried out related explorations around various thermoplastics, such as polyurethane, styrene ethylene butylene styrene (SEBS), cyclic olefin polymers and copolymers.
3. Standard setting
From the whole industry chain perspective of research and development, products, and enterprises, the pain points that restrict the rapid development of organ chips also include the ununiformed standards of each organ chip, the specificity of detection, the lack of horizontal comparison but only self-comparison, the need for a third-party agency or consortium to develop uniform standards. At present, the United States is making an industry alliance and has designed three organ-on-a-chip testing centers. At the same time, the automated and standardized testing instruments for organ chips will also be the focus of the future development of organ chips.
References:
1. Brancato V, Oliveira JM, Correlo VM, Reis RL, Could 3D models of cancer enhance drug screening, Biomaterials, 232, 119744 (2020).
2. Gould, S. E., Junttila, M. R. & de Sauvage, F. J. Translational value of mouse models in oncology drug development, Nat. Med. 21, 431–439 (2015).
3. Killion, J. J., Radinsky, R. & Fidler, I. J. Orthotopic models are necessary to predict therapy of transplantable tumors in mice, Cancer Metastasis Rev. 17, 279–284 (1999).
4. Waring, M. J. et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies, Nat. Rev. Drug Discov. 14, 475-486 (2015).
5. Ma C, Peng Y, Li H, Chen W, Organ-on-a-Chip: A New Paradigm for Drug Development, Trends Pharmacol Sci, 42, 119-133 (2021).
6. Bhatia, S. N. & Ingber, D. E. Microfluidic organs-on-chips, Nat. Biotechnol. 32, 760–772 (2014).
7. Lucie A. Low, Christopher P. Austin, Organs-on-chips: into the next decade, Nat Rev Drug Discov,20,345-361 (2021)
8. Park SE, Georgescu A, Huh D, Organoids-on-a-chip, Science, 364, 960-965 (2019).
9. Kasendra, M. et al, Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci. Rep. 8, 2871 (2018).
10. Organ on Chip in Development (ORCHID), EU – H2020 project grant agreement no 766884 (AMD-766884-3).
11. Bonaventura, A., Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19, Nat Rev Immunol, 21, 319-329 (2021).
12. Zhang H, Wang CY, Zhou P, Yue H, Du R, Histopathologic Changes and SARS-CoV-2 Immunostaining in the Lung of a Patient With COVID-19. Ann Intern Med, 173, 323-324 (2020).